Fizzy Graphene
September 25, 2017
One of the few attractions of
Upstate New York is the accessibility of
venues for
outdoor sporting activities. It's a great place to be if you're into
hunting,
fishing,
skiing,
hiking, and
camping, but one major concern is the number of
snowmobile accidents and
fatalities.[1] My
family lived in Upstate New York when I was a
child, and we went on camping excursions
summers to some of
New York's many state parks.

Walden Pond (left) was made famous by Henry David Thoreau (1817-1862) in his book, Walden; or, Life in the Woods.
Thoreau lived at Walden for two years to experience the spiritual benefits of a simplified lifestyle. This lifestyle, however, would not have been possible without the charity of his friends and family.
(Walden Pond in November, 2009, photo by John Phelan, via Wikimedia Commons
While the
swimming was fun, I was further
entertained by hanging-out with the other
teenagers who were there with their families. These came from all over the state, other states, and
Canada, so their
customs and
particular manner of speech were a little weird. In my
city,
carbonated beverages were called "soda," while teenagers from the
western part of the state called them "pop." This observation of mine is verified in a study by
Matthew Campbell and
Greg Plumb of
East Central University, Oklahoma, who created a
map of this near-
dichotomy for the entire US.[2]
Whether called "soda," or "pop," such soft drinks are
flavored and
sweetened carbonated water, which is
water infused with
carbon dioxide gas. If water doesn't
react with the gas, the
absorption of a gas in water is described by
Henry's Law,
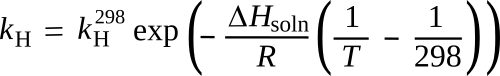
![]()
In this
equation, the Henry's law
constant,
kH is equal to the
ratio of the
concentration of of the gas in water
ca to its
partial pressure in
equilibrium above the water
pg; that is,
kH = ca/pg. The
ΔHsoln is the
enthalpy of solution,
R is the
gas constant, and
kH298 is the value at
standard temperature, which is actually 298.15°
C.
In order to maximize the amount of carbon dioxide dissolved into carbonated water, the process is done at a
temperature just above the
freezing point of water. When your
bottles of soda (or pop) is opened at a higher temperature, some of the gas begins to escape, as can be seen by the
bubbly effervescence. While a mild reaction of carbon dioxide with water does produce a small amount of
carbonic acid, H2CO3, to produce a slightly
sour taste, the main effect of the carbonation is the
transport of
aroma molecules up your
nose, and a tingle on your
tongue, a
sensation in the category of
mouthfeel.
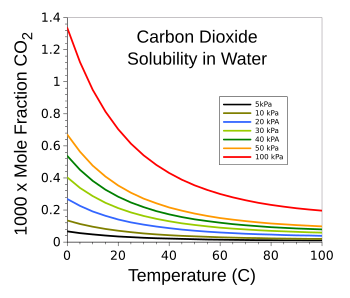
Solubility of carbon dioxide in water.
Not surprisingly, because of Henry's Law, greater partial pressure and lower temperature leads to a higher solubility.
(Graphed using Gnumeric from data in ref. 3. Click for larger image.)[3]
![]() Materials scientists
Materials scientists from the
Department of Materials Science and Engineering and the
Frederick Seitz Materials Research Laboratory of the
University of Illinois at Urbana-Champaign have found a method for production of large area
graphene sheets using carbonated water at a critical step in the process.[4-5] This
blog has a few articles about graphene. Here are the ones from just this year:
• Graphene from Ethylene, June 5, 2017
• Soybean Graphene, March 23, 2017
• Graphene Friction, February 9, 2017
• Graphene Putty, January 16, 2017
Graphene is usually
synthesized by
chemical vapor deposition onto a
copper substrate that acts as a
catalyst.[5] A principal problem is the release and transfer of the graphene from the copper to an
insulating substrate. That's the critical step addressed by
SungWoo Nam, an
assistant professor of mechanical science and engineering at Illinois, and
his research team, who developed a cleaner and more
environmentally friendly method for this release using carbonated water.[5] Graphene transfer allows the reuse of the copper catalyst substrate.[4]
Although they're not scientifically accurate, everyone likes an artist's conception of an idea.
The active phrase here is "de gustibus non disputandum est"(About taste, there can be no question).
(University of Illinois image.)
The carbonic acid in the carbonated water allows an
electrochemical reduction of the
cuprous oxide (Cu2O) interlayer between the copper catalyst substrate and the graphene, and the subsequent delamination of the graphene.[4-5] Previous methods used less environmentally-friendly
salt/
alkali-based
electrolytes.[4] An important next step is the use of
food-grade ethyl cellulose as a transfer layer. Ethyl cellulose is an inexpensive and environmentally-friendly alternative to the typically used
polycarbonate or PMMA (
poly methyl methacrylate), both of which need
toxic solvents for their removal.[4-5]
Says team member,
Michael Cai Wang, a PhD student and lead researcher on the project,
"In our case, we are using a bio-mass derived polymer, ethyl cellulose, for the coating... A common and inexpensive polymer often used as a food additive, ethyl cellulose is solvated in just ethanol. This not only makes our graphene transfer process more environmentally friendly, it is now also compatible with a variety of polymeric and soft biological materials such as common plastics and hydrogels that would otherwise not tolerate harsh solvents... After you transfer the graphene, the carbonic acid simply evaporates away as carbon dioxide and water, which doesn’t require any further rinsing."[5]
While graphene has yet to leave the
laboratory and become a high-volume
electronic material, it's always good to look ahead to the time when the
health of
workers in a
manufacturing environment needs to be protected. Since electronic materials are greatly affected by
chemical impurities, this carbon dioxide process is a means to produce extremely pure materials. Carbon dioxide processing might also be a viable alternative for other processes that require delamination.[5]
Funding for this research was provided by the
National Science Foundation, the
Air Force Office for Scientific Research,
NASA's Space Technology Research Grants Program, and the
Natural Sciences and Engineering Research Council of Canada.[5]
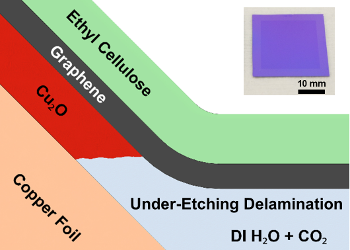
Schematic diagram of the graphene process, with the end result depicted in the insert.
Cuprous oxide has a large exothermic free energy of formation, so an oxide layer is naturally present at a copper surface.
(University of Illinois image.)
![]()
References:
- New York State Parks Recreation and Historic Preservation, Snowmobile Unit, 2015-2016 Season Report, April 22, 2016 (PDF File).
- Kayla Burns, I call it Pop, You call it Soda, Smithsonian Collections Blog, July 28, 2011.
- Solubility of Carbon dioxide in Water at Various Temperature and Pressure, Colorado State University Web Site.
- Michael Cai Wang, Widianto P. Moestopo, Satoshi Takekuma, Shama Farabi Barna, Richard T. Haasch, and SungWoo Nam, "A sustainable approach to large area transfer of graphene and recycling of the copper substrate," Journal of Materials Chemistry C (Advance Article, 2017), DOI: 10.1039/C7TC02487H.
- Mike Koon, "Fizzy" soda water could be key in clean and sustainable manufacture of "flat" wonder material: graphene," University of Illinois Press Release, August 16, 2017.
Linked Keywords: Upstate New York; sport venue; outdoor recreation; outdoor sporting activities; hunting; fishing; skiing; hiking; camping; snowmobile; accident; fatalities; family; child; summer; New York's many state parks; Walden Pond; Henry David Thoreau (1817-1862); Walden; or, Life in the Woods; spirituality; spiritual; charity; friendship; friend; John Phelan; Wikimedia Commons; swimming; entertainment; entertain; adolescence; teenager; Canada; social norm; custom; dialect; manner of speech; city; soft drink; carbonated beverage; Western New York; Matthew Campbel; Greg Plumb; East Central University, Oklahoma; map; dichotomy; flavor; flavored; sweetness; sweetened; carbonated water; water; carbon dioxide; gas; chemical reaction; react; absorption; Henry's Law; equation; physical constant; ratio; concentration; partial pressure; chemical equilibrium; enthalpy of solution<; gas constant; standard conditions for temperature and pressure; standard temperature; Celsius; temperature; melting point; freezing point; bottle; bubble; effervescence; carbonic acid, H2CO3; sour taste; diffusion; transport; aroma compound; aroma molecule; nose; tongue; sensory nervous system; sensation; mouthfeel; solubility of carbon dioxide in water; solubility; Gnumeric; materials scientist; >Department of Materials Science and Engineering; Frederick Seitz Materials Research Laboratory; University of Illinois at Urbana-Champaign; graphene; chemical synthesis; synthesize; chemical vapor deposition; copper; substrate; catalysis; catalyst; electrical insulator; SungWoo Nam; assistant professor; research team; environmentally friendly; science; scientific; artist; portrayal; conception; idea; de gustibus non disputandum est; electrochemistry; electrochemical; redox; reduction; copper(I) oxide; cuprous oxide (Cu2O); acid salt; alkali; electrolyte; food additive; food-grade; ethyl cellulose; polycarbonate; poly methyl methacrylate; toxicity; toxic; solvent; biomass; bio-mass; polymer; coating; ethanol; biomaterial; biological material; plastic; hydrogel; evaporation; evaporate; laboratory; electronic material; health; worker; manufacturing; manufacturing environment; chemical impurity; funding of science; National Science Foundation; Air Force Office for Scientific Research; NASA; National Space Grant College and Fellowship Program; Space Technology Research Grants Program; Natural Sciences and Engineering Research Council of Canada; schematic diagram; chemical process; exothermic reaction; standard Gibbs free energy of formation.