High Entropy Ceramics
February 26, 2024
One
principle that
scientists and
engineers learn is the difference between the
ideal and the
actual. Unfortunately, the difference is learned mostly in the prolonged process of discovery of why your
circuit or
experiment is not giving the
anticipated response.
Electrical resistance changes with
temperature,
materials expand and contract, and that ideal
amplifier you're using has
noise, limited
frequency response, and a significantly small
input impedance. Eventually, one learns to anticipate such problems, and
computer simulations are now available as a
design aid.
An ideal
elemental metal is a
perfect crystal; but, just
melting and
solidifying a metal does not give you a huge
single crystal. Instead, you get a
lump of
polycrystals, and the
physical properties of that lump are not just
dependent on the metal, but also the
microstructure of the
solid. When making an
alloy, a
liquid mixture of several different types of metals will give you a
polycrystalline solid consisting of multiple crystal
phases when solidified. The crystal phases present in an alloy and their relative
concentrations can be used to increase its
strength by limiting the movement of
dislocations.
As I wrote in a
previous article (High-Entropy Alloys, June 20, 2016), multiple metals can be combined to make an alloy with just a single phase. In 2014,
materials scientists at
Oxford University produced a single phase alloy of equal portions of five elements.[1] This alloy was a
face-centered cubic solid solution with
composition Fe20Cr20Mn20Ni20Co20. Such a material is known as a
high entropy alloy.
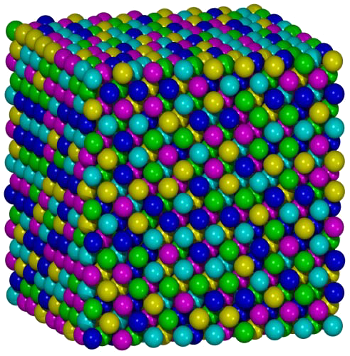
Atomic structure model of the Oxford University Fe20Cr20Mn20Ni20Co20 alloy.
In this image, magenta = Fe, green = Co, blue = Cr, cyan = Ni, and yellow = Mn. Aside from slight differences in atomic size, swapping colors would serve just as well, since the composition has equal numbers of each element.
(Image by Shaoqing Wang, via Wikimedia Commons.)
Do high entropy alloys have any properties that are especially useful? High entropy alloys have been shown to have better
radiation resistance.[2] Now that
fossil fuel energy is being replaced by other
technologies, their potential for use in
nuclear reactors could become important.
Energetic particles, such as
neutrons, cause
displacement of
atoms from their positions in the
crystal lattice upon
impact and produce
lattice defects and
dislocations that affect the alloy's
mechanical properties. The peculiar structure of a high-entropy alloy diminishes the severity of defect formation.
High entropy ceramics are a companion material type of high entropy alloys, and they have radiation resistant properties as well. The first studied
classes of these HECs are the high entropy
carbides (HECs) and
oxides (HEOs).[3] Such ceramics can exhibit better stability at high temperature, better
corrosion resistance, and significantly higher
hardness, than metallic alloys.[3] Early
studies of HECs have reported that radiation effects, such as
void formation,
amorphization, and radiation-induced
segregation do not occur.[3] One high entropy
carbide, (
CrNbTaTiW)
C, demonstrated considerable radiation immunity.[3] This carbide was not amorphized under radiation dosage conditions at which high entropy alloys are likewise unaffected.[3]
C.png)
Radiation resistance of the high entropy carbide, CrNbTaTiW)C, compared with a high entropy alloy at the same radiation dosage. The histograms show the average diameter of bubbles formed from xenon irradiation at the same dosage of 10 displacements-per-atom. (A portion of fig 4 of ref. 3,[3] released under a Creative Commons license.)
High-entropy carbides were discovered in 2018.[4-5] Now a research team from
Duke University (Durham, North Carolina),
Penn State University (State College, Pennsylvania, the
Missouri University of Science and Technology (Rolla, Missouri),
North Carolina State University (Raleigh, North Carolina)y, and the
State University of New York at Buffalo (Buffalo, New York) has developed a method, called a disordered
enthalpy–entropy descriptor (DEED), that aids the discovery of
synthesizable multicomponent ceramics.[4] DEED assists in
experimental discovery of single phase high entropy
carbonitrides and
borides with a focus on the use of
sintering by
hot isostatic pressing.[5] In sintering by hot isostatic pressing,
powders of the constituent compounds of the ceramic are heated in a
vacuum at high temperature and
pressure for many
hours.[5]
The DEED method has already predicted 900 new synthesizable ceramics, seventeen of which have been prepared and
analyzed.[5] The ceramics synthesized by this sintering process are dark grey or black, and they have a
density close to that of a metal alloy; however, they are hard and
brittle as are other ceramics.[5] The Duke University research team maintains a materials
database called the Duke Automatic-FLOW for Materials Database (AFLOW) that offer data for
simulation methods such as DEED.[6] AFLOW even contains data on an
exotic material,
HfPt3 investigated by some of my
graduate school colleagues in the
1970s. This research was
funded by
United States Department of Defense Multidisciplinary University Research Initiative (MURI), and the United States Department of Defense
High Performance Computing Modernization Program (HPC-Frontier).[5]

The description of entropy as a function of the number of microstates possible in an ideal gas was discovered by Austrian physicist, Ludwig Boltzmann (1844-1906), in 1877. His famous equation, now written as S = kBln(Ω), in which S is the entropy, kB is the Boltzmann constant, and Ω is the number of microstates, appears on his grave marker at the Central Cemetery in Vienna, Austria. (Left image, and right image, both from Wikimedia Commons. Click for larger image.)
References:
- B. Cantor, I.T.H. Chang, P. Knight, and A.J.B. Vincent, "Microstructural development in equiatomic multicomponent alloys," Materials Science and Engineering, vols. 375-377 (July 2004), pp. 213-218, doi:10.1016/j.msea.2003.10.257.
- O. El-Atwani, N. Li, M. Li, A. Devaraj, J. K. S. Baldwin, M. M. Schneider, D. Sobieraj, J. S. Wróbel, D. Nguyen-Manh, S. A. Maloy, and E. Martinez, "Outstanding radiation resistance of tungsten-based high-entropy alloys," Science Advances, vol. 5, no. 3 (March 1, 2019), DOI: 10.1126/sciadv.aav2002.
- Matheus A. Tunes, Stefan Fritze, Barbara Osinger, Patrick Willenshofer, Andrew M. Alvarado, Enrique Martinez, Ashok S. Menon, Petter Strö, Graeme Greaves, Erik Lewin, Ulf Jansson, Stefan Pogatscher, Tarik A. Saleh, Vladimir M. Vishnyakov, and Osman El-Atwani, "From high-entropy alloys to high-entropy ceramics: The radiation-resistant highly concentrated refractory carbide (CrNbTaTiW)C," Acta Materialia, vol. 250 (May 15, 2023), Article no. 118856, https://doi.org/10.1016/j.actamat.2023.118856. This is an open source article with a PDF file available at the same URL.
- Simon Divilov, Hagen Eckert, David Hicks, Corey Oses, Cormac Toher, Rico Friedrich, Marco Esters, Michael J. Mehl, Adam C. Zettel, Yoav Lederer, Eva Zurek, Jon-Paul Maria, Donald W. Brenner, Xiomara Campilongo, Suzana Filipović, William G. Fahrenholtz, Caillin J. Ryan, Christopher M. DeSalle, Ryan J. Crealese, Douglas E. Wolfe, Arrigo Calzolari, and Stefano Curtarolo, "Disordered Enthalpy-Entropy Descriptor for High-Entropy Ceramics Discovery," Nature, vol. 625, no. 7993 (January 4, 2024), pp. 66-73DOI: 10.1038/s41586-023-06786-y. This is an open source article with a PDF file available at the same URL.
- Computational method discovers hundreds of new ceramics for extreme environments, Duke University Press Release, January 3, 2024.
- AFLOW Website, Center for Autonomous Materials Design, Materials Science, Duke University.
Linked Keywords: Principle; scientist; engineer; theory of forms - ideal state; ideal; reality; actual; electronic circuit; experiment; expectation (epistemic); anticipated response; electrical resistance; temperature; material; thermal expansion; expand and contract; amplifier; noise (electronics); frequency response; electrical impedance; input impedance; computer simulation; design; chemical element; elemental; metal; single crystal; perfect crystal; melting; freezing; solidifying; lump; crystallite; polycrystal; physical property; dependent variable; microstructure; solid; alloy; liquid; mixture; polycrystalline; phase (matter); concentration; strength of materials; dislocation; materials science; materials scientist; University of Oxford; Oxford University; cubic crystal system; face-centered cubic; solid solution; empirical formula; Iron; Fe; Chromium; Cr; Manganese; Mn; Nickel; Ni; Cobalt; Co; high entropy alloy; space-filling model; atomic structure model; atomic radius; atomic size; chemical element; radiation damage; radiation resistance; fossil fuel; energy; technology; technologies; nuclear reactor; energy; energetic; subatomic particle; neutron; displacement (vector); crystal structure; crystal lattice; impact (mechanics); crystallographic defect; lattice defect; mechanical properties; high entropy oxide; high entropy ceramic; categorization; class; carbide; oxide; corrosion resistance; hardness; research; study; porosity; void formation; amorphous solid; amorphization; segregation (materials science); carbide; Niobium; Nb; Tantalum; Ta; Titanium; Ti; Tungsten; W; Carbon; C; entropy; radiation dosage; histogram; diameter; bubble; xenon; irradiation; Creative Commons license; Duke University (Durham, North Carolina); Penn State University (State College, Pennsylvania; Missouri University of Science and Technology (Rolla, Missouri); North Carolina State University (Raleigh, North Carolina); State University of New York at Buffalo (Buffalo, New York); enthalpy; chemical synthesis; synthesizable; carbonitride; boride; sintering; hot isostatic pressing; powder (substance); vacuum; pressure; hour; analysis; analyze; density; brittleness; brittle; database; computer simulation; simulation method; exotic; HfPt3; graduate school; colleague; 1970s; funding of science; funded; United States Department of Defense; Multidisciplinary University Research Initiative (MURI); High Performance Computing Modernization Program (HPC-Frontier); function (mathematics); microstate (statistical mechanics); ideal gas; Austria; Austrian; physicist; Ludwig Boltzmann (1844-1906); Boltzmann constant; headstone; grave marker; Vienna, Austria.