Graphene Putty
January 16, 2017
There are many examples of
materials research producing a different product than what was intended. One example is
WD-40, an
oil originally designed as a
corrosion-barrier coating for
metal, especially the
stainless steel fuel tanks of the
Atlas missile.[1] WD-40 is now an ubiquitous
household penetrating lubricant used for extracting
rusted bolts and many other applications. My favorite "
off-label" use is the removal of
adhesive residue, especially from old
duct tape, on compatible materials. The
WD-40 Web Site lists many more uses.[2]
The composition of WD-40 is a
trade secret, but its
ingredients,
as disclosed on Wikipedia, are as follow:
• 50% Aliphatic hydrocarbons.
• 25%, or less, mineral oil or light lubricating oil.
• 12-18% low vapor pressure aliphatic hydrocarbons. These reduce the low-viscosity to allow for spray application, and they evaporate quickly.
• 2-3% carbon dioxide, used as a flammability-retarding propellant.
• 10%, or less, inert ingredients.
Dupont chemist,
Stephanie Kwolek, was looking for a lightweight substitute for
steel wire in
radial tires when she discovered the
ultra-strong polymer,
Poly-paraphenylene terephthalamide, known as
Kevlar, which has a
tensile strength five times that of steel.
Belgian-
American chemist,
Leo Baekeland (1863-1944), was searching for a
synthetic replacement for the
natural product,
shellac, when he discovered
Bakelite, the first synthetic
thermosetting plastic. His "
insoluble product of
phenol and
formaldehyde" was commonly used as an
electrical insulator.[3]
Modern chemists are
safety conscious, and they wear
protective gloves when working with
chemicals. This wasn't the case in 1879 when
Constantin Fahlburg noticed a
sweet taste on his
hand after working with
coal tar reactions. He had discovered
saccharin, the first
artificial sweetener. Saccharin became important when
sugar supply became difficult during
World War I, and it was important for
sugar-free diets in later years.
The
Eastman Kodak company, the giant of
photography before its chemical phase was superseded by
digital photography, did more than adhere
silver halide to
film. Its
Kodak Research Laboratories did
research in many chemical areas, and it was there that Kodak
engineer,
Harry Coover discovered
cyanoacrylate "super-glue", a material originally tried as a clear plastic for
gun sights. This product was originally sold as "
Eastman 910."
As I wrote in an earlier article (
Silly Putty, August 6, 2014), research on a
natural rubber substitute during
World War II led to development of
Silly Putty® based on a mixture of
polydimethylsiloxane, [C
2H
6OSi]
n, and
silicone oil reacted with
boric acid.[4-5] Silly Putty® itself is a
trademarked name of a material sold by
Crayola, but similar materials sold under different names.[6] More than four thousand
tons of Silly Putty have been sold since 1950.[7]
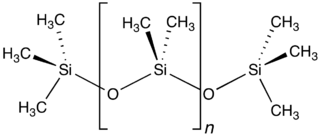
The structure of polydimethylsiloxane.
(Via Wikimedia Commons.)
Silly Putty has the unusual
mechanical characteristic that it can be slowly worked, like a
clay, but it behaves as an
elastic solid when the
rate of applied
force is large. This characteristic is a result of the
viscoelasticity of the polydimethylsiloxane.
According to Wikipedia, this is the composition of Silly Putty:
Dimethyl siloxane polymer, terminated with boric acid, 65%
Silica (crystalline quartz), 17%
Thixatrol ST (castor oil derivative), 9%
Polydimethylsiloxane, 4%
Decamethyl cyclopentasiloxane, 1%
Glycerine, 1%
Titanium dioxide, 1%
Silly putty is the basis of a novel
sensor material created by
physicists at the
School of Physics of
Trinity College Dublin (Dublin, Ireland) and the
University of Manchester (Manchester, UK). Silly putty mixed with
graphene exhibits an
electrical conductivity that varies with applied
force.[8-9] This research was enabled by a the €1 billion
Graphene Flagship initiative.[9] This research team has just
published their results in
Science.[8]
While graphene has been used as an additive in
nanocomposites, its behavior in highly
viscoelastic polymer
matrices has not been well characterized.[8] When graphene is added to silly putty, forming a material the research team calls "G-putty," the lightly
cross-linked polysilicone, its electromechanical properties are changed substantially.[8-9] There's a
post-deformation temporal relaxation of
electrical resistance and a nonmonotonic change in
resistivity with
strain.[8]
The
gauge factor of the material, the
ratio of the resistance change to the strain, is greater than 500. This compares with about 2-5 for metals, 30 for
polysilicon, and up to 100 for
thick film resistors of special compositions. The sensitivity is such that a force as small as a
spider's footstep can be detected.[9] While spider detection would be a niche application, the material could prove useful as a sensor for
respiration,
pulse rate, and
blood pressure.[9]
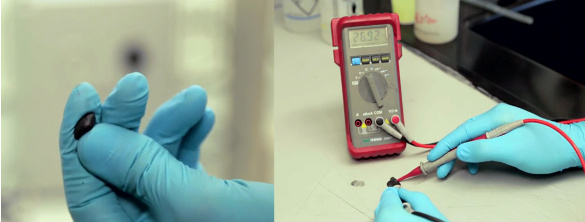
Graphene-filled silly putty (G-putty) is soft and flexible. It also exhibits a large resistance change with applied pressure. (Still images from a Trinity College Dublin video.)
While conductivity changes like this usually arise from
percolation, the electrical properties of G-putty appear to be associated with nanosheets of graphene moving in the
low-viscosity polymer matrix.[8] The research team was able to devise a
mathematical model that describes the electromechanical properties of G-putty.[8]
Says
Jonathan Coleman, a
professor at Trinity College Dublin,
"When we added the graphene to the silly putty, it caused it to conduct electricity, but in a very unusual way. The electrical resistance of the G-putty was very sensitive to deformation with the resistance increasing sharply on even the slightest strain or impact. Unusually, the resistance slowly returned close to its original value as the putty self-healed over time."[9]

Study co-author, Jonathan Coleman, and son, Oisin, playing with types of silly putty.
In Coleman's case, it's graphene-filled silly putty, G-putty.
(Trinity College Dublin image.)
References:
- WD-40 History, WD-40 Company, Inc., Web Site.
- List of 2000+ Uses of WD-40, WD-40 Company, Inc., Web Site (PDF File).
- Leo H Baekeland, "Method of making insoluble products of phenol and formaldehyde," US Patent No. 942,699, December 7, 1909.
- Mcgregor Rob Roy and Warrick Earl Leathen, "Treating dimethyl silicone polymer with boric oxide," US Patent No. 2,431,878, December 2, 1947.
- James G E Wright, "Process for making puttylike elastic plastic, siloxane derivative composition containing zinc hydroxide," US Patent No. 2,541,851, Feb 13, 1951.
- Maurice A. Minuto, "Method of Making Bouncing Silicone Putty-Like Compositions," US Patent No. 4371493, February 1, 1983.
- Eugene S. Robinson, "How That Pinkish Goo Called Silly Putty Came Out Of Its Shell," NPR, July 17, 2014.
- Conor S. Boland, Umar Khan, Gavin Ryan, Sebastian Barwich, Romina Charifou, Andrew Harvey, Claudia Backes, Zheling Li, Mauro S. Ferreira, Matthias E. Möbius, Robert J. Young, and Jonathan N. Coleman, "Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites," Science, vol. 354, no. 6317 (December 9, 2016), pp. 1257-1260, DOI: 10.1126/science.aag2879.
- State of the art sensors made from graphene and children's toy silly putty, Trinity College Dublin Press Release, December 8, 2016.
Permanent Link to this article
Linked Keywords: Materials science; materials research; WD-40; lubricant; oil; anti-corrosion; corrosion-barrier; coating; metal; stainless steel; fuel tank; Atlas missile; household; penetrating oil; penetrating lubricant; rust; rusted; bolt; off-label; adhesive; duct tape; trade secret; ingredient; Wikipedia; aliphatic hydrocarbon; mineral oil; vapor pressure; low-viscosity; aerosol spray; evaporation; evaporate; carbon dioxide; flame retardant; flammability-retarding; propellant; excipient; inert ingredient; Dupont; chemist; Stephanie Kwolek; steel; wire; radial tire; strength of materials; ultra-strong; polymer; Poly-paraphenylene terephthalamide; Kevlar; tensile strength; Belgium; Belgian; America; American; Leo Baekeland (1863-1944); chemical synthesis; synthetic; natural product; shellac; Bakelite; thermosetting polymer; thermosetting plastic; solubility; insoluble; phenol; formaldehyde; electrical insulator; safety; nitrile rubber; protective gloves; chemical compound; Constantin Fahlburg; sweetness; sweet taste; hand; coal tar; chemical reaction; saccharin; sugar substitute; artificial sweetener; sucrose; sugar; World War I; sugar-free diet; Eastman Kodak company; photography; digital photography; silver halide; film; Kodak Research Laboratories; research; engineer; Harry Coover; cyanoacrylate "super-glue"; gun sight; Eastman 910; natural rubber; World War II; Silly Putty®; polydimethylsiloxane; silicone oil; boric acid; trademark; Crayola; ton; Wikimedia Commons; mechanical characteristic; clay; elasticity; elastic; solid; rate; force; viscoelasticity; Dimethyl siloxane; polymer; terminate; boric acid; silicon dioxide; Silica; crystal; crystalline; quartz; castor oil; derivative; Decamethyl cyclopentasiloxane; Glycerol; Glycerine; Titanium dioxide; sensor; physicist; School of Physics; Trinity College Dublin (Dublin, Ireland); University of Manchester (Manchester, UK); graphene; electrical conductivity; force; Graphene Flagship initiative; scientific literature; publish; Science; nanocomposite; viscoelasticity; viscoelastic; composite material; matrix; cross-link; siloxane; polysilicone; deformation; temporal relaxation; electrical resistance; resistivity; strain; gauge factor; ratio; polysilicon; thick film resistor; spider; respiration; pulse rate; blood pressure; pressure; percolation theory; mathematical model; Jonathan Coleman; professor; co-author; son; Leo H Baekeland, "Method of making insoluble products of phenol and formaldehyde," US Patent No. 942,699, December 7, 1909; Mcgregor Rob Roy and Warrick Earl Leathen, "Treating dimethyl silicone polymer with boric oxide," US Patent No. 2,431,878, December 2, 1947; James G E Wright, "Process for making puttylike elastic plastic, siloxane derivative composition containing zinc hydroxide," US Patent No. 2,541,851, Feb 13, 1951; Maurice A. Minuto, "Method of Making Bouncing Silicone Putty-Like Compositions," US Patent No. 4371493, February 1, 1983.