Superamphiphobic Paper
June 10, 2013
Paper is one of the most common
consumer materials. I can't write that it's
the most common, since we each buy close to a hundred
pounds of another material almost every week. If you haven't guessed, that's the
gasoline for our
automobiles, since a
gallon of gasoline
weighs about six pounds.
Although this falls short of the "
pint's a pound" standard,
hydrocarbon liquids have about the same
density, so they weigh about the same, as the following table shows.
Since paper is ubiquitous, and it has quite a few desirable properties, I've written quite a few articles about it in the past (
Paper Chase, September 7, 2010,
Paper Accelerometer, March 10, 2011,
Crumpled Paper, October 25, 2011, and
Crumpling, August 27, 2012). Although the demise of paper as a
display medium has been predicted, it doesn't look as if that will happen anytime soon (
Electronic Paper, November 4, 2010).
One of the more useful properties of paper is its ability to
absorb liquid, and
paper towels and
facial tissues are the major paper products entering my home. I may buy a
ream or two of
printer paper every six
months; but, in that case, absorbency is a disadvantage, since I spill my
coffee far too often. Paper is sometimes
coated to inhibit the absorption of water and other fluids.
It's possible to reduce water absorption of surfaces without the addition of a coating. A surface can be
microstructured to exhibit the
lotus effect, appropriately named after the particular surface microstructure of the water-repellent
lotus leaf. The lotus leaf is
Superhydrophobic; that is, its surface has excellent water repellent properties. The superhydrophobicity arises from microscopic surface protrusions that force a high
contact angle for any
droplet.

Leaf of an Indian Lotus, Nelumbo nucifera. (Portion of a photograph taken in Kolkata, West Bengal, India, by J.M. Garg, via Wikimedia Commons.)
Superhydrophobicity has gained renewed interest, since
nanoscale technology has facilitated rapid nanotexturing of surfaces. Five of my articles in 2012 were about superhydrophobicity (
Tiny Droplets, March 6, 2012,
Superhydrophobic Anti-Glare Glass, May 2, 2012,
Icing-Resistant Surfaces, August 8, 2012,
Droplet Logic, September 19, 2012,
Bubbles and Boiling, September 24, 2012, and
Super Condensing Surfaces, November 9, 2012)
It's time to add a new
adjective to our portfolio of surface effects. If we replace the "hydro" part of superhydrophobic with "amphi," from the
Greek adjective,
αμφι, meaning "both," we get
superamphiphobic. The both in this case refers to
polar liquids, such as water, and
non-polar liquids, such as
oil. A superamphiphobic material will resist both water and oil; or, in other words, it will be both hydrophobic and
oleophobic.
Scientists from the
School of Chemical and Biomolecular Engineering and the
Institute of Paper Science and Technology at the
Georgia Institute of Technology have combined
oxygen plasma etching and
fluoropolymer deposition to create superamphiphobic paper.[1-2] They were able to achieve liquid drop contact angles greater than 150° for water,
ethylene glycol,
motor oil, and
n-hexadecane.[1]
The paper is made by the creation of surface patterns on both the micrometer and nanometer size scales, followed by a thin fluoropolymer coating.[2] The starting materials are standard
softwood and
hardwood fibers used in papermaking, and the process is as follows:[2]
• The cellulose fibers are broken up through a mechanical grinding process.
• As in traditional papermaking, the fibers are then pressed in the presence of water and the water is removed.
• Butanol, which inhibits the hydrogen bonding between cellulose fibers, is used for additional processing. This allows better control of the cellulose fiber distribution than the water processing.
• The outer, amorphous layer of the paper is removed by an oxygen plasma etch. This exposes the crystalline cellulose nanofibrils.
• A thin fluoropolymer coating is applied.
The oxygen plasma etch uncovers smaller cellulose structures, and this adds a second level of surface texture needed for the lotus effect.
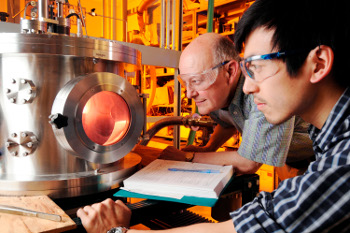
Monitoring an oxygen plasma etch are Dennis Hess, a professor in the Georgia Tech School of Chemical and Biomolecular Engineering, and graduate research assistant Lester Li.
(Georgia Tech photograph by Gary Meek.)
Rigid surfaces have been textured by lithography to be
superamphiphobic. Hydrophobicity is easiest to achieve, since water has a high
surface tension. However, the lower surface tension oils need undercut features to cause
re-entrant angles between the surface and droplets.[2] The superamphiphobic paper has been made in samples that are about four
inches on a side. However, the process can be scaled up.[2]
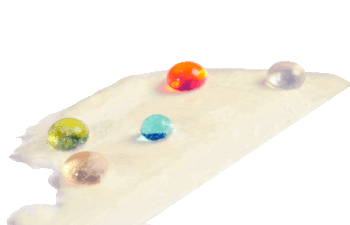
Droplets of water, motor oil, ethylene glycol and n-hexadecane on a superamphiphobic paper sample.
(Georgia Tech photograph by Gary Meek.)
Dennis Hess, a professor in the Georgia Tech School of Chemical and Biomolecular Engineering, who teamed on this project with Lester Li, a graduate research assistant, and
Victor Breedveld, an associate professor in the Georgia Tech School of Chemical and Biomolecular Engineering, had this to say.
"We believe this is the first time that a superamphiphobic surface - one that repels all fluids - has been created on a flexible, traditional and heterogeneous material like paper."[2]
One application for such paper is a "lab-on-a-sheet," an inexpensive
biomedical diagnostic device in which liquid samples would flow along printed channels to combine with
antigens and
reagents. Says Hess,
"We have shown that we can do the operations necessary for a microfluidic device... We can move the droplet along a pattern, split the droplet and transfer the droplet from one piece of paper to another. We can do all of these operations on a two-dimensional surface."[2]
References:
- Lester Li, Victor Breedveld and Dennis W. Hess, "Design and Fabrication of Superamphiphobic Paper Surfaces," ACS Appl. Mater. Interfaces, Article ASAP, May 6, 2013, DOI: 10.1021/am401436m.
- John Toon, "Advanced Paper Could be Foundation for Inexpensive Biomedical and Diagnostic Devices," Georgia Tech Press Release, May 28, 2013. Also on the Georgia Tech School of Chemical and Biomolecular Engineering Web Site, and the Georgia Tech Institute of Paper Science and Technology Web Site.
Permanent Link to this article
Linked Keywords: Paper; consumer; material; pound; gasoline; automobile; gallon; weight; United States customary units; fluid volume; pint's a pound; hydrocarbon; liquid; density; Hexane; Octane; Olive Oil; Decane; Gasoline; Pentane; Kerosene; Toluene; Turpentine; Benzene; Fuel oil; Water; display device; display medium; electronic paper; absorption; absorb; paper towel; facial tissue; ream; printer; month; coffee; coating; coated; microstructure; lotus effect; lotus; leaf; superhydrophobe; superhydrophobic; contact angle; droplet; Nelumbo nucifera; Wikimedia Commons; nanoscopic scale; nanoscale; technology; adjective; Greek language; chemical polarity; polar; non-polar; oil; lipophobicity; oleophobic; School of Chemical and Biomolecular Engineering; Institute of Paper Science and Technology; Georgia Institute of Technology; oxygen; plasma etching; fluoropolymer; physical vapor deposition; ethylene glycol; motor oil; n-hexadecane; softwood; hardwood; fiber; cellulose; butanol; hydrogen bond; hydrogen bonding; amorphous solid; crystallinity; crystalline; Dennis Hess; Gary Meek; surface tension; convex and concave polygons; re-entrant angle; inch; Victor Breedveld; biomedical; diagnosis; diagnostic; antigen; reagent.