Tiny Droplets
March 6, 2012
Even when we ignore the
chemistry of the
liquid itself, there's much interesting
science in a
droplet. Ignoring the
mechanics of the droplet fluid, there are still two important properties of liquid droplets. The first is the
surface tension, which affects the
contact angle of a droplet on a surface. The second is the observation that the ratio of the
surface area to the
volume of a
sphere is a function of 1/r, where r is the
radius. This means that surface effects are extremely important as droplets become smaller in size.

Since
evaporation rate depends on the surface area, this means that a volume of liquid partitioned into many small droplets will evaporate much faster than when it's collected into a single large droplet. That's what makes a spill of mercury dangerous. The surface tension of mercury is very high, 485 mN/m, as compared to 72 mN/m for water, so the liquid breaks into many small droplets when it impacts a hard surface, as can be seen in the figure.
As the world shifts into nano-mode, much more research is being done on small droplets. One study, by scientists at
Princeton University's Department of Mechanical and Aerospace Engineering, the
Institut Jean le Rond d'Alembert (Paris) and the
University of Paris, has investigated how droplets wet
flexible fibers. This process is important in both natural systems, such as
foliage and
avian plumage, and industrial systems that include fibrous media.[1-2]
The research team investigated the common case of droplets sitting on adjacent fibers. In their experiments, they clamped flexible
glass fibers at one end, placed droplets at the clamped end, and recorded the droplets as they moved to the free end. The mechanical properties of the fibers are important, since their high aspect ratios, combined with their
elasticity, allows the surface tension of the droplets to draw the fibers together when they roll to areas with narrower gap.
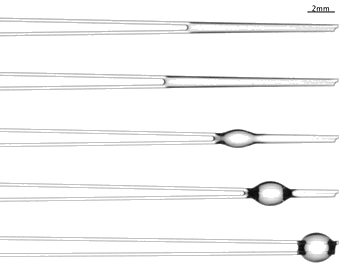
Oil drops on flexible fibers.
Droplets below a critical size will spread between the fibers, but larger droplets are sessile on the fibers.
Princeton University image by Camille Duprat and Suzie Protière.
(Used with permission)
Small droplets pulled the fibers together to wet them along long segments, but it was found that there was a critical droplet volume beyond which this process did not occur; that is, the droplets remained intact, sitting atop the fibers (see photograph).[1-2]
Said
Howard Stone, a coauthor of a paper on this study that appeared in
Nature,
"If in any engineering problem you can learn an optimal size above which something does not happen, you have learned something very important about the system."[1]
Just about every process in the world relates to minimizing
energy, and the droplet-fiber system is no exception. droplets spread when the liquid has less
surface energy in forming a film between the fibers than remaining as a drop.[1] This research, sponsored by
Unilever, can have application for the production of optimal
hairspray delivery systems; or, in the
self-assembly of micro- and nano-structures.[1]
Another research team, from the
Massachusetts Institute of Technology Department of Mechanical Engineering and the
Stokes Institute, the
University of Limerick (Limerick, Ireland), has been investigating the condensation and evaporation of droplets from
textured surfaces; specifically,
superhydrophobic surfaces.[3-5] The textures are designed to favor the formation of suspended droplets atop nanostructures. The suspended droplets have minimal contact to the surface, which promotes shedding. Shedding would otherwise occur at a characteristic
capillary length.[4] This is an application of
Cassie's law, which expresses the fact that rough surfaces will support droplets with high apparent contact angle, thereby enhancing
hydrophobicity.
Thw mechanism for droplet detachment from a surface without nanostructure requires the growth of the droplet to a size at which
gravity or other forces overcome the surface tension that holds the droplet on the surface. This mechanism often proceeds by adjacent droplets merging to form a larger droplet.[3]
Faster droplet shedding would aid to removal of
heat from surfaces, but droplets sitting on nanostructures have reduced contact with the surface, so
heat transfer is impeded. The MIT-Limerick study investigated the heat transfer as it is affected by the surface contact of droplets. The research team imaged droplets using
environmental scanning electron microscopy, and they found that in condensation the initial droplet growth rates of partially wetting droplets were six times larger than that of droplets suspended on nanopillars.[4]
The partially wetting droplets sit on nanopillars and wet just a small area of the surface, but this action is highly dependent on the size, spacing, width-to-height ratios and nanoscale roughness of the pillars.[3] Partially wetting droplets had a four-six times higher heat transfer rate than that of droplets suspended at the pillar tips. The nanostructured surfaces had about a fifty percent enhancement of heat removal, compared with flat hydrophobic surfaces, when the droplets were partially wetting; and about a seventy percent degradation of heat removal when the droplets were suspended.[3-4]

A mix of droplet types and sizes on a nanotextured surface. (Screen capture from a YouTube video))
Such research is important to improvements in many processes, including
desalination plants and the many applications requiring heat transfer from solids to liquids. Such heat transfer applications include systems that use
solar energy to evaporate water. Obvious applications are cooling
semiconductor circuits and improvement of
heat pipes. The research was supported by the
U.S. Department of Energy,[3] and the results are published in the journal,
ACS Nano.[4]
References:
- John Sullivan, "Less is more: Study of tiny droplets could have big applications," Princeton University Press Release, February 23, 2012.
- C. Duprat, S. Protière, A. Y. Beebe and H. A. Stone, "Wetting of flexible fibre arrays," Nature, vol. 482, no. 7386 (February 23, 2012), pp. 510-513.
Supplementary Movie for Ref. 2, showing the evolution of a drop of two microliters volume on a rail formed by two fibres of 4 centimeter length, separated by 0.76 mm (11.5Mb file).
- David L. Chandler, "New nanopatterned surfaces could improve the efficiency of powerplants and desalination systems," MIT News Office Press Release, February 23, 2012.
- Nenad Miljkovic, Ryan Enright and Evelyn N. Wang, "Effect of Droplet Morphology on Growth Dynamics and Heat Transfer during Condensation on Superhydrophobic Nanostructured Surfaces," ACS Nano, vol. 6,no. 2 (January 31, 2012), pp 1776-1785.
- Nenad Miljkovic, "Making Nanodroplets Drop Faster," (Music by Alastair Cameron), YouTube Video by MITNewsOffice, Feb 22, 2012. "MIT mechanical engineering graduate student Nenad Miljkovic discusses his team's work on condensation, nanodroplet formation, and new nanopatterned surfaces that could boost the efficiency of power plants and desalination systems."
Permanent Link to this article
Linked Keywords: Chemistry; liquid; science; droplet; mechanics; surface tension; contact angle; surface area; volume; sphere; radius; evaporation; mercury; surface tension of mercury; millinewton; mN; meter; mercury droplets; NIH; nanotechnology; Princeton University; Department of Mechanical and Aerospace Engineering; Institut Jean le Rond d'Alembert (Paris); University of Paris; deflection; flexible; fiber; foliage; avian plumage; glass fiber; elasticity; Howard Stone; Nature; energy; surface energy; Unilever; hairspray; self-assembly; Massachusetts Institute of Technology; MIT; Department of Mechanical Engineering; Stokes Institute; University of Limerick (Limerick, Ireland); texture; superhydrophobic; capillary action; capillary; Cassie's law; hydrophobe; hydrophobicity; gravitation; gravity; heat; heat transfer; environmental scanning electron microscopy; YouTube; desalination; solar energy; semiconductor circuit; heat pipe; U.S. Department of Energy; ACS Nano; Camille Duprat; Suzie Protière.