Predicting Metallic Glass Formation
May 28, 2018
The recent passing of
theoretical physicist,
Stephen Hawking, on March 14, 2018, reminds us that many people with
physical disabilities have contributed to
human culture. Possibly the earliest example is the
blind poet,
Homer, who composed the
Iliad and the
Odyssey.
Mathematician,
Leonhard Euler (1707-1783), had impaired vision
from age 30, and he was nearly blind at age sixty. His response to such disability was merely, "
Now I will have fewer distractions," and his
mathematical output was essentially undiminished.
German physicist,
Gustav Kirchhoff (1824-1887), had limited mobility that required his use of
crutches and a
wheelchair for most of his life, but that didn't stop him from developing
five significant discoveries; namely,
Kirchhoff's circuit laws,
Kirchhoff's law of thermal radiation,
Kirchhoff equations of fluid dynamics,
Kirchhoff's three laws of spectroscopy, and
Kirchhoff's law of thermochemistry. I wrote about Kirchhoff in an
earlier article (Kirchhoff–Plateau Problem, June 15, 2017).
An experimental physicist in his natural abode, the laboratory.
Here, Kirchhoff is using a spectrometer of his own design.
(An illustration from the History of Physics by Poul la Cour and Jacob Appel, 1896, via Wikimedia Commons.)
Preminent
electrical engineer,
Charles Proteus Steinmetz (1865-1923), had the multiple
birth defects of
dwarfism,
hunchback, and
hip dysplasia. Despite these physical disabilities, Steinmetz was a
prodigy in
physics and
mathematics. After his 1889 emigration to the
United States, he discovered how
magnetic hysteresis of
transformer cores contributes to
electrical power losses. While working at
General Electric, Steinmetz designed the generators for the
Niagara Falls power-generation station. I wrote about Steinmetz in an
earlier article (Charles Proteus Steinmetz, May 3, 2012).

Charles Proteus Steinmetz (1865-1923).
Charles Proteus Steinmetz was born as Carl August Rudolph Steinmetz on April 9, 1865, in Breslau (now, Wrocław, Poland).
Upon emigration, Steinmetz changed his name, deciding that "Charles" sounded more American that "Carl." The added "Proteus" was a reference to the god of Greek mythology, Proteus (Πρωτευς), who would foretell the future to anyone who captured him, but he would change shape to avoid being captured. As a theorist, Steinmetz could be said to have foretold the future.
Since Steinmetz's father and grandfather had birth defects similar to his own, he decided he would never marry.
(Wikimedia Commons image)
Deafness afflicted American
inventor,
Thomas Alva Edison (1847-1931), some of whose many accomplishments I reviewed in several previous articles (
Edison's Iron Mine, September 20, 2010,
People Who Live in Concrete Houses..., June 30, 2011,
His Master's Voice, August 15, 2011, and
Edison's Nickel-Iron Battery Modernized, July 9, 2012). Deafness also afflicted
biochemist and
Nobel Laureate,
Edwin Krebs (1918-2009), who
collaborated on the identification of
phosphorylation as the way that a small quantity of
hormone can produce a large bodily effect.[1]
English materials scientist and
metallurgist,
William Hume-Rothery (1899-1968), was deaf, his total hearing lose caused by a
viral infection just before his entering
college in 1917. This didn't prevent him from achieving a
First-Class Honours degree in
chemistry as an
undergraduate and his subsequent studies for his
Ph.D. at the
Royal School of Mines. In 1926, he devised his
Hume-Rothery rules as an aid to determining how
metals will mix to form
alloys.[2] This was one of the first attempts at alloy system
modeling.
Hume-Rotherey realized the importance of
atomic size in the
crystallization of
materials. Chemically similar
metals (chemically-similar defined as having the same
crystal structure with atoms having the same
valency and nearly equal
electronegativity) will enter into
substitutional solid solution if the size of their atoms differ by no more than 15%. If their size differs by more than this, there's a likelihood that
secondary phases will form, some of which are detrimental to
alloy strength. This is more likely when the electronegativity difference is large.
As we know from
steelmaking,
carbon easily dissolves in
iron, but carbon atoms are very much smaller than iron atoms. The reason for this is that carbon is not substitutional, but
interstitial; that is, the carbon atoms position themselves between iron atoms rather than replacing the iron atoms. Hume-Rothery's rule for interstitial substitution is that the atomic radius of the interstitial
solute atoms should be less than about 60% the atomic radius of the
solvent atoms. These Hume-Rothery rules, if proposed today, this would be an excellent
data mining exercise.
This leads us to the problem of how to define atomic size.
Xray diffraction allows us to examine the spacing between atoms in
crystals to assess the atomic radii; but, as we find, the spacing does not give an identical atomic radius for the same
chemical element in different crystals. It depends on such factors as the valence state, whether the
chemical bonding in the crystal is
covalent or
ionic, and the number of neighboring atoms, which is called the
coordination number. In order to "
compare apples with apples," we need to use the proper atomic radii for the elements in our alloys.
Fortunately, some
scientists have created tables that list the atomic radii for all these variations, the most popular of which is the listing of Shannon and Prewitt, which I've used in my own work.[3-4] As noted on
Google Scholar, the principal Shannon paper has 43,526
citations. Today, these
data are available online in an easily accessible format.[5] Example data for
hafnium, with radii in
Angstrom units, appear below.
Atomic radius and electronegativity are simplistic concepts that give guidance, but there are more effective modeling techniques for prediction of alloy phases. In the 1970s, I designed
computer models in which
thermodynamic free energy was used to predict alloy phases. Since such thermodynamic data were limited, many
approximations and
rules of thumb were required. One such rule is
Trouton's rule, which states that the
entropy of vaporization, which is the
enthalpy of vaporization divided by the normal
boiling point, is about 75
J/
mol-
K.
As everyone has noticed,
artificial intelligence is taking over the world, and it's been applied to the problem of predicting what compositions of elements will yield
metallic glasses. In a similar fashion to the alloy-forming rules that I mentioned above, metallic glass compositions have been discovered using
empirical rules, such as Turnbull's rule that metallic glasses form near deep
eutectics in the alloy
phase diagrams. Metallic glasses are
amorphous,
non-crystalline solids. Since some compositions exhibit low
magnetic hysteresis, they're used to make
energy-efficient transformers with an amorphous metal core. Some metallic glasses have a high
magnetic permeability, so they are very effective
electromagnetic shielding materials.
Scientists from the
SLAC National Accelerator Laboratory (Menlo Park, California),
Northwestern University (Evanston, Illinois), the
University of Chicago (Chicago, Illinois), the
University of South Carolina (Columbia, South Carolina), the
University of New South Wales (Sydney, Australia), and the
National Institute of Standards and Technology (Gaithersburg, Maryland) have applied
machine learning techniques to the task of discovering metallic glasses. They report their progress in a
free, open access paper in
Science Advances.[6-8]
The motivation for this approach is the slow rate of progress to discovery of new metallic glass compositions by the traditional empirical techniques. The
periodic table is teaming with elements, leading to numerous combinations that should yield millions of metallic glasses. However, only about 6,000 have been discovered.[7-8] They are hard to find, since they often contain three or more elements, and they are sensitive to
processing technique.[6]
Such machine-learning requires an initial
dataset. The
research team used data from 6780
melt spinning experiments at 5313 unique compositions in the
Landolt-Börnstein handbook, a compendium of fifty years of such data. This handbook contains 315 binary systems (25% of the possible binaries of the 51 examined elements) and spot data from 293 different ternary systems, with a bias towards data for the
Al-
La-
Ni ternary.[6] As can be expected, this dataset is
biased towards positive results, with 71% of listed experiments leading to amorphous metals.[6]
The research team combined taught a machine learning algorithm about conditions favorable to metallic glass formation with this initial dataset, and then proceeded with
iterative high-throughput experiments to refine the
algorithm.[6] The improved model was able to refine predictions for the
Co-
V-
Zr alloy system, and it eventually discovered of metallic glasses in two previously unreported ternaries.[6] Says paper
co-author,
Apurva Mehta of SLAC, "We were able to make and screen 20,000 (compositions) in a single year."[7-8]
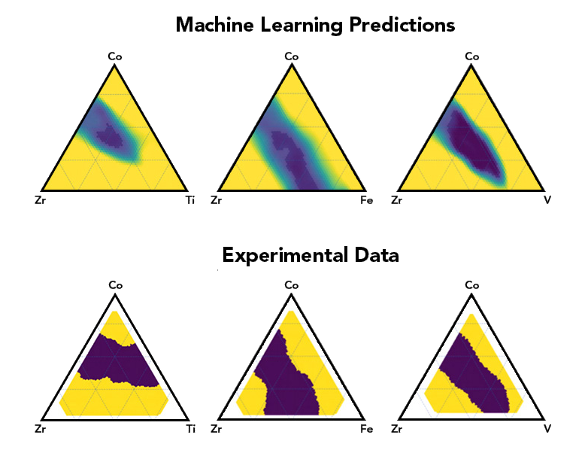
Metallic glass, machine learning predictions vs experiment. (SLAC National Accelerator Laboratory image by Yvonne Tang.)
Says
Jason Hattrick-Simpers, a materials research
engineer at NIST and an author of the paper,
"One of the more exciting aspects of this is that we can make predictions so quickly and turn experiments around so rapidly that we can afford to investigate materials that don’t follow our normal rules of thumb about whether a material will form a glass or not... AI is going to shift the landscape of how materials science is done, and this is the first step."[7-8]
This artificial intelligence approach has paid off, since the discovery rate for metallic glass increased from about 0.25% of samples tested to nearly 50%.[7-8] This study was funded by the
US Department of Energy, the
Center for Hierarchical Materials Design at Northwestern University, and the National Institute of Standards and Technology.[7-8]
References:
- Neeraja Sankaran, "Scientists With Disabilities Must Confront Societal As Well As Physical Challenges," The Scientist, January 23, 1995.
- W. Hume-Rothery, "Research on the nature, properties and conditions of formation of intermetallic compounds, with special reference to certain compounds of tin," J. Inst. Metals., vol 35 (1926), pp. 295-307.
- R. D. Shannon, "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides," Acta Crystallographica Section A, vol. 35, no. 5 (September, 1976), pp. 751-767.
- R. D. Shannon and C. T. Prewitt, "Revised values of effective ionic radii," Acta Cryst., vol. B26, no, 7 (July 15, 1970), pp. 1046-1048, https://doi.org/10.1107/S0567740870003576.
- Database of Ionic Radii, Atomistic Simulation Group, Materials Department, Imperial College.
- Fang Ren, Logan Ward, Travis Williams, Kevin J. Laws, Christopher Wolverton, Jason Hattrick-Simpers and Apurva Mehta, "Accelerated discovery of metallic glasses through iteration of machine learning and high-throughput experiments," Science Advances, vol. 4, no. 4 (April 13, 2018), Article no. eaaq1566, DOI: 10.1126/sciadv.aaq1566. This is an open access article with a PDF file here.
- Artificial intelligence accelerates discovery of metallic glass, Northwestern University Press Release, April 13, 2018.
- Glennda Chui, "Scientists Use Machine Learning to Speed Discovery of Metallic Glass," SLAC Press Release, April 13, 2018.
Linked Keywords: Theoretical physics; theoretical physicist; Stephen Hawking; physical disability; human culture; visual impairment; blindness; poet; Homer; Iliad; Odyssey; mathematician; Leonhard Euler (1707-1783); eyesight deterioration; Now I will have fewer distractions; mathematical; Germany; German; physicist; Gustav Kirchhoff (1824-1887); crutch; wheelchair; Kirchhoff's circuit laws; Kirchhoff's law of thermal radiation; Kirchhoff equations of fluid dynamics; Kirchhoff's three laws of spectroscopy; Kirchhoff's law of thermochemistry; experimental physics; experimental physicist; laboratory; spectrometer; Wikimedia Commons; electrical engineering; electrical engineer; Charles Proteus Steinmetz (1865-1923); congenital disorder; birth defect; dwarfism; kyphosis; hunchback; hip dysplasia; child prodigy; physics; mathematics; United States; magnetic hysteresis; transformer; magnetic core; electrical power loss; General Electric; Niagara Falls power-generation station; Wrocław, Poland; emigration; American; Greek mythological figure; god; Greek mythology; Proteus; theory; theorist; father; grandfather; invention; inventor; Thomas Alva Edison (1847-1931); biochemist; Nobel Prize in Physiology or Medicine; Nobel Laureate; Edwin Krebs (1918-2009); collaboration; collaborate; phosphorylation; hormone; English; metallurgy; metallurgist; William Hume-Rothery (1899-1968); viral disease; viral infection; college; First-Class Honours degree; chemistry; undergraduate; Doctor of Philosophy; Ph.D.; Royal School of Mines; Hume-Rothery rules; metal; alloy; mathematical model; modeling; atom; atomic; crystallization; material; crystal structure; valence electron; valency; electronegativity; substitutional solid solution; crystal; secondary phase; strength; steelmaking; carbon; iron; interstitial compound; solute; solvent; data mining; X-ray crystallography; Xray diffraction; chemical element; chemical bond; covalent bond; ionic bond; coordination number; apples and oranges; scientist; Google Scholar; citation; data; hafnium; Angstrom unit; charge; covalent radius; ionic radius; computer model; thermodynamic; Gibbs free energy; approximation; rules of thumb; Trouton's rule; entropy of vaporization; enthalpy of vaporization; boiling point; joule; mol; kelvin; artificial intelligence; aqmorphous metal; metallic glass; empirical; eutectic; phase diagram; amorphous; non-crystalline solid; magnetic hysteresis; energy conversion efficiency; energy-efficient; amorphous metal transformer; transformers with an amorphous metal core; magnetic permeability; electromagnetic shielding; SLAC National Accelerator Laboratory (Menlo Park, California); Northwestern University (Evanston, Illinois); University of Chicago (Chicago, Illinois); University of South Carolina (Columbia, South Carolina); University of New South Wales (Sydney, Australia); National Institute of Standards and Technology (Gaithersburg, Maryland); machine learning; free, open access paper; Science Advances; periodic table; processing; dataset; research; melt spinning; experiment; Landolt-Börnstein handbook; aluminium; lanthanum; nickel; statistical hypothesis testing; bias towards positive results; iteration; iterative; high-throughput screening; high-throughput experiments; algorithm; cobalt; vanadium; zirconium; author; Apurva Mehta; amorphous metal; metallic glass; machine learning; experiment; Yvonne Tang; Jason Hattrick-Simpers; engineer; materials science; United States Department of Energy; Center for Hierarchical Materials Design at Northwestern University.