Michael Faraday
October 8, 2018
Scientists are honored in many ways. Noted scientists are recipients of
prizes and
awards for their work, but they may also have prizes and awards named in their honor. Some examples of this in
physics are the
Arthur L. Schawlow Prize in Laser Science, the
Hans A. Bethe Prize, the
Irving Langmuir Prize in Chemical Physics, the
Max Delbruck Prize in Biological Physics, and the
Maria Goeppert Mayer Award, awarded by the
American Physical Society. The
cross-disciplinary Langmuir prize is awarded in odd years by the American Physical Society, and in even years by the
American Chemical Society.
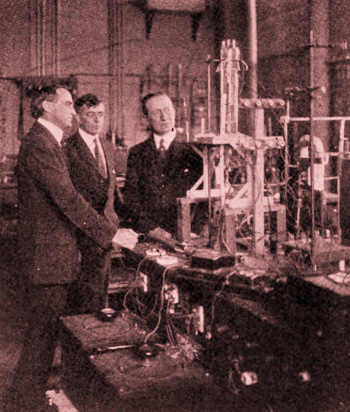
Left to right, Willis R. Whitney, Director of the General Electric Research Laboratory, Irving Langmuir, and Guglielmo Marconi, August, 1922.
They're looking at a recently developed 20 kilowatt triode vacuum tube.
Both Langmuir and Marconi were Nobel Laureates, Langmuir in chemistry and Marconi in physics.
(Wikimedia Commons image, modified for artistic effect.)
The American Chemical Society has its own such awards, examples being the
Priestley Medal, the
Glenn T. Seaborg Award for Nuclear Chemistry, the
Irving Langmuir Award in Chemical Physics, and the
Peter Debye Award in Physical Chemistry. The
Institute of Electrical and Electronics Engineers has the
James Clerk Maxwell Medal, jointly awarded with the
Royal Society of Edinburgh, the
John von Neumann Medal, the
Charles Proteus Steinmetz Award, the
Marie Sklodowska-Curie Award, and the
Edison Medal. Likewise, computer scientists of the
Association for Computing Machinery have their
A.M. Turing Award, the
Eckert Mauchly Award, the
Grace Murray Hopper Award, and the
Gordon Bell Prize.
An even greater honor goes to the many scientists who have
measurement units named after them. Here's a partial list, somewhat organized by subject area (Most units derive from physics).
Physics
• Planck unit (a "natural unit") - Max Planck
• joule (J, energy) - James Prescott Joule
• newton (N, force) - Isaac Newton
• tesla (T, magnetic flux density) - Nikola Tesla
Chemistry
• kelvin (K, temperature) - William Thomson, Lord Kelvin
• pascal (Pa, pressure) - Blaise Pascal
• coulomb (C, electric charge) - Charles-Augustin de Coulomb
• dalton (Da, atomic mass) - John Dalton
Electrical Engineering
• ampere (A, electric current) - André-Marie Ampère
• volt (V, electric potential) - Alessandro Volta
• ohm (Ω, electrical resistance) - Georg Ohm
• watt (W, power) - James Watt
Computer Science
• baud (Bd, symbol rate) - Emile Baudot
• shannon (Sh, information) - Claude Shannon
Often Seen in the Popular Press
• Degree Fahrenheit (°F, temperature) - Daniel Gabriel Fahrenheit
• Mach number (Ma, relative speed) - Ernst Mach
• Richter magnitude (earthquake power) - Charles Francis Richter
• Scoville unit (chili pepper capsaicin content) - Wilbur Scoville

Ohm's law illustrated graphically as Georg Ohm equals Alessandro Volta over André-Marie Ampère.
Ohm's law is usually written R = E/I, although V is sometimes used to signify voltage instead of E.
(Source images, Ohm, Volta, and Ampère, from Wikimedia Commons. Click for larger image.)
Perhaps the greatest honor is to have your name associated with a
chemical element.
Fifteen elements have been named to honor sixteen scientists (
Curium was named to honor both
Marie Sklodowska-Curie and
Pierre Curie). Examples are
einsteinium (
Albert Einstein),
fermium (
Enrico Fermi),
mendelevium (
Dmitri Mendeleev),
rutherfordium (
Ernest Rutherford), and
copernicium (
Nicolaus Copernicus). Notably,
seaborgium was named after
Glenn T. Seaborg, the only person to have an element named for him in his lifetime.

Glenn T. Seaborg in the laboratory. From the surroundings, you can tell that he's a chemist and not a physicist.
Seaborg was awarded the 1951 Nobel Prize in Chemistry for his work on the synthesis and identification of ten transuranic elements.
Seaborg died on February 25, 1999, but element-106, Seaborgium (Sg), was named in his honor in 1997. Politics is everywhere, even in the sciences, so Seaborg's honor came as a result of a compromise in the naming of elements 104-108.
(Lawrence Berkeley National Laboratory/United States Department of Energy image, via Wikimedia Commons)
One scientist who deserves special mention is
Michael Faraday (1791-1867). Faraday, who had little formal
education, made significant discoveries in physics and chemistry, including the discovery of
benzene, the
invention of the first
electric generator, and the invention of an early version of the
Bunsen burner. He is also responsible for the
terminology for
electrode,
ion,
anode, and
cathode. It's no wonder that there are
many things that bear Faraday's name. Faraday is mentioned in more than 1,900 papers on
arXiv.

Michael Faraday (1791-1867).
Faraday became became an apprentice to a bookbinder and bookseller at age 14.
In his seven year apprenticeship he read many books and developed an interest in the sciences, being especially enthralled by electricity.
(From the Project Gutenberg book, Great Britain and Her Queen by Annie E. Keeling, after a photograph by Henry Dixon & Son Ltd., via Wikimedia Commons)
![]() Farad
Farad
The farad (f) is the unit of capacitance. Until late in the 20th century, when supercapacitors were introduced, a capacitance of one farad would fill most of a room. Fortunately, most electronic circuitry requires capacitance values of less than a millionth of a farad, a microfarad, while power supply filters use capacitance values of about a thousandth farad, or 1000 microfarad.
Faraday Constant
The Faraday constant (F) is the electric charge in a mole of electrons, which is 96485.33 coulombs per mole. This unit is commonly used in electroplating, since it allows a calculation of the amount of material deposited.
Faraday's law of induction
Faraday's law of induction relates electricity and magnetism by quantifying the effect of passing a permanent magnet through a coil of wire, or the equivalent operation of a time-varying magnetic field, as in a transformer. It states that the induced electromotive force in an electrical circuit is equal to the negative of the time rate of change of the magnetic flux enclosed by the circuit.
Faraday Cage
A Faraday cage is a conductive enclosure that blocks electromagnetic fields. It was invented by Faraday in 1836. The enclosure need not be a continuous conductor, and it might be a conductive metal mesh with openings somewhat smaller in size than the longest wavelength that needs to be blocked. The shield of a coaxial cable is a common example of a Faraday cage. A static magnetic field, such as the Earth's magnetic field, can't be blocked by a Faraday cage.
Faraday Cup
A close cousin to the Faraday cage is the Faraday cup. As its name implies, it's a cup made from an electrical conductor, usually metal, that captures charged particles in a vacuum. The electrical current passing through the cup allows counting of the number of ions or electrons collected in the cup. This calculation, of course, uses the Faraday constant.
Faraday Effect
The Faraday effect is the rotation of the plane of polarization of light as it passes through a magneto-optical material. Bismuth-substituted iron garnets, with which I work for many years, exhibit a very large Faraday effect, and they are often used in optical isolators. Single crystals of terbium gallium garnet are used in optical isolators in high-powered laser systems.
Faraday Wave
Faraday waves are the standing wave patterns that appear on the surface of vibrating liquids, and they were first described by Faraday in 1831. Faraday waves form interesting patterns that have the forms of stripes, close-packed hexagons, squares, and quasi-repeating patterns, so they resemble the Chladni patterns seen on the surface of a vibrating plate. The cause of these waves is a hydrostatic instability when the vibration frequency is greater than a critical value.
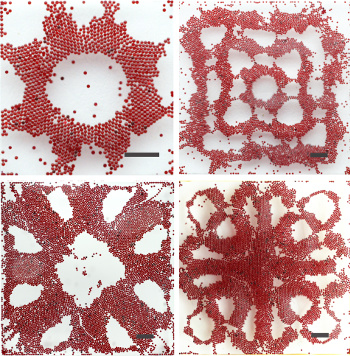
Faraday wave patternsFaraday wave patterns created by excitation of 200-micrometer polystyrene beads on a liquid surface. The scale bar is 2 millimeters.
The pattern is formed by the migration of beads to nodal regions on the liquid surface.
(Portions of a Wikimedia Commons image by "Faraday Telsa." Click for larger image.)
Faraday's journey to scientific greatness began in 1813 as a chemical assistant to
Humphrey Davy, who
isolated several new elements by
electrochemical separation, at the
Royal Institution. Faraday was associated with the Royal Institution for the rest of his life, performing
experiments and giving
public lectures. He was made a member of the
Royal Society in 1824; and, a year later, he became director of its
laboratory.
Frank A. J. L. James, a
professor at
University College London and a professor of the
history of science at the Royal Institution, has written a short history of the Royal Institution in an
article in a recent issue of
Physics Today.[1] This article contains an overview of Faraday's time there. James is also the
author of the 2010 book,
Michael Faraday: A Very Short Introduction,[2] and the
editor of the six-volume collection,
The Correspondence of Michael Faraday (1991–2012).[3]
An article in the August/September 2001 issue of
APS News marked the
anniversaries of two important dates in Faraday's studies in electromagnetism.[4] These are September 4, 1821 and August 29, 1831, the former date marking his discovery of the principle of the
electric motor, and the later date marking his discovery of the principle of the
dynamo that led to simple electric generators.
Alan Hirshfeld, a professor of physics at the
University of Massachusetts Dartmouth, has written a
biography of Faraday,
The Electric Life of Michael Faraday,[5] which was reviewed in the January, 2007, issue of Physics Today.[6]
References:
- Frank A. J. L. James, "The legacies of the Royal Institution," Physics Today, vol. 71, no. 8 (August 1, 2018), pp. 36ff., https://doi.org/10.1063/PT.3.3996.
- Frank A. J. L. James, "Michael Faraday: A Very Short Introduction," Oxford University Press (December 16, 2010), 144 pages, ISBN-13: 978-0199574315 (via Amazon).
- Frank A. J. L. James, "Correspondence of Michael Faraday, Volume 1, 1811-1831, The Institution of Engineering and Technology (June 30, 1991), 724 pages, ISBN-13: 978-0863412486 (via Amazon).
- This Month in Physics History - September 4, 1821 and August 29, 1831: Faraday and Electromagnetism, APS News, vol. 10, no. 8 (August/September 2001).
- Alan Hirshfeld, "The Electric Life of Michael Faraday," Walker Books (March 7, 2006), 256 pages, ISBN-13: 978-0802714701 (via Amazon).
- Cyrus Hoffman, "Book Review: The Electric Life of Michael Faraday," Physics Today, vol. 60, no. 1 (January 1, 2007), p. 57, https://doi.org/10.1063/1.2709560.
Linked Keywords: Scientist; prize; award; physics; Arthur L. Schawlow Prize in Laser Science; Hans A. Bethe Prize; Irving Langmuir Prize in Chemical Physics; Max Delbruck Prize in Biological Physics; Maria Goeppert Mayer Award; American Physical Society; cross-disciplinary; American Chemical Society; Willis R. Whitney; General Electric Research Laboratory; Irving Langmuir; Guglielmo Marconi; kilowatt; triode; vacuum tube; Nobel Laureate; chemistry; physics; Wikimedia Commons; Priestley Medal; Glenn T. Seaborg Award for Nuclear Chemistry; Peter Debye Award in Physical Chemistry; Institute of Electrical and Electronics Engineers; James Clerk Maxwell Medal; Royal Society of Edinburgh; John von Neumann Medal; Charles Proteus Steinmetz Award; Marie Sklodowska-Curie Award; Edison Medal; Association for Computing Machinery; A.M. Turing Award; Eckert Mauchly Award; Grace Murray Hopper Award; Gordon Bell Prize; scientific units named after people; Planck unit (a "natural unit"); Max Planck; joule (J, energy); James Prescott Joule; newton (N, force); Isaac Newton; tesla (T, magnetic flux density); Nikola Tesla; kelvin (K, temperature); William Thomson, Lord Kelvin; pascal (Pa, pressure); Blaise Pascal; coulomb (C, electric charge); Charles-Augustin de Coulomb; dalton (Da, atomic mass); John Dalton; electrical engineering; ampere (A, electric current); André-Marie Ampère; volt (V, electric potential); Alessandro Volta; ohm (Ω, electrical resistance); Georg Ohm; watt (W, power); James Watt; computer science; baud (Bd, symbol rate); Emile Baudot; shannon (Sh, information); Claude Shannon; mass media; popular press; Degree Fahrenheit (°F, temperature); Daniel Gabriel Fahrenheit; Mach number (Ma, relative speed); Ernst Mach; Richter magnitude (earthquake power); Charles Francis Richter; Scoville unit (chili pepper capsaicin content); Wilbur Scoville; Graphical Ohm's law; Ohm's law; Georg Ohm; voltage; chemical element; fifteen elements have been named to honor sixteen scientists; curium; Marie Sklodowska-Curie; Pierre Curie; einsteinium; Albert Einstein; fermium; Enrico Fermi; mendelevium; Dmitri Mendeleev; rutherfordium; Ernest Rutherford; copernicium; Nicolaus Copernicus; seaborgium; Glenn T. Seaborg; chemist; physicist; Nobel Prize in Chemistry; synthesis; transuranic element; element-106, Seaborgium (Sg); politics; science; chemical elements naming controversy; compromise; Lawrence Berkeley National Laboratory; United States Department of Energy; Michael Faraday (1791-1867); education; benzene; invention; electric generator; Bunsen burner; terminology; electrode; ion; anode; cathode; things named after Michael Faraday; arXiv; apprenticeship; apprentice; bookbinding; bookbinder; bookselling; bookseller; electricity; Project Gutenberg; Great Britain and Her Queen by Annie E. Keeling; farad (f); capacitance; 20th century; eElectric double-layer capacitor; supercapacitor; room; electronics; electronic circuit; power supply; filter; Faraday constant (F); electric charge; mole; electron; electroplating; calculation; material; Faraday's law of induction; magnetism; permanent magnet; solenoid; coil of wire; magnetic field; transformer; electromotive force; electrical circuit; negative number; magnetic flux; Faraday cage; electrical conductor; conductive; electromagnetic field; metal; mesh; wavelength; electromagnetic shielding; shield; coaxial cable; Earth's magnetic field; Faraday Cup; cousin; charged particle; vacuum; electrical current; Faraday effect; rotation; plane of polarization; light; magneto-optic effect; bismuth; iron garnet; optical isolator; single crystal; terbium gallium garnet; power (physics); high-power; laser system; Faraday wave; standing wave pattern; surface; acoustics; vibrating; liquid; stripe (pattern); hexagonal tiling; close-packed hexagons; square; Chladni patterns; plane (geometry); plate; hydrostatic; instability; frequency; micrometer; polystyrene; bead; scale bar; millimeter; node (physics); nodal regions; Humphrey Davy; chemical element discovery; electrolysis; electrochemical separation; Royal Institution; experiment; public lecture; laboratory; Frank A. J. L. James; professor; University College London; history of science; academic publishing; article; Physics Today; author; editor; APS News; anniversary; electric motor; dynamo; Alan Hirshfeld; University of Massachusetts Dartmouth; biography.