Negative Thermal Expansion
December 12, 2016
Among the useful items in my
electronics toolbox are various lengths of
heat-shrink tubing. As every electronics
technician knows, covering a
cable connection with a short piece of heat-shrink tubing gives your work a finished appearance; or, as my
father would say, it "hides all the
sins." Heat-shrink tubing also offers
strain-relief at the junctions of cables and
connectors.
Heat-shrink tubing was
invented in 1962.[1]
Thermoplastics such as
PTFE,
PVC and
polyolefin are formed into
tubes and their
molecules are
cross-linked, typically through
electron radiation. The tubes are then expanded in
diameter by application of a differential
pressure between the inside and outside of the tubes while they're held above the thermoplatic's
crystalline melting point. The hot tubes are rapidly
quenched to
room temperature, and this locks in the larger diameter.
At the time of use, heating the tubing above the crystalline melting point with a
hot air gun causes it to shrink back to its smaller diameter. In most electronics applications, the tubing is polyolefin, which is stable from -55°C to 135°C, and it will shrink about half in diameter. Thermal shrinkage of the thermoplastic,
polystyrene, was the basis of the 1970s
Shrinky Dinks craft toys.

A Shrinky Dinks butterfly.
(Portion of a Wikimedia Commons image.)
These are extreme examples of
temperature-induced changes in a
material's size, and the changes are
irreversible under subsequent heating or cooling. The vast majority of materials, however, undergo a
reversible thermal expansion, increasing in size as the temperature is increased, and returning to their normal size upon cooling. Such positive thermal expansion is the norm, but there are a few materials that show a negative thermal expansion. I reviewed thermal expansion in an
earlier article (Negative Thermal Expansion, November 11, 2011).
Thermal expansion is relatively
linear within short ranges of temperature, so it's quantified by a linear
thermal expansion coefficient, alpha (α),
α = (1/L)(∂L/∂T)
for which L is an arbitrary gauge length, T is the temperature. The thermal expansion coefficient is quite different between materials, as the following table of the room temperature (20°C) coefficient shows. Notice that hydrogen-bonded liquids (ethanol and water) have a much larger thermal expansion coefficient than other materials.
Thermal expansion is simply the consequence of
atomic vibrational energy acting against the
bonding forces that hold the atoms in crystals and
molecules together. That's why negative thermal expansion (NTE) is so unusual.
Water has NTE between freezing and 3.984°C; but, as a hydrogen bonded material, something unusual like this might be expected. However, the mainstay of our electronic circuitry,
silicon exhibits NTE at very low temperatures, 18 - 120 K. A more useful NTE material is cubic zirconium tungstate, ZrW2O8, which exhibits NTE from close to absolute zero up to 775 °C.[2-3]
Since hafnium is chemically similar to zirconium, and
molybdenum is chemically similar to tungsten,
analogs of cubic zirconium tungstate with such substitutions exhibit NTE also. NTE in another material was discovered in 2010.
Scandium fluoride (ScF3) was found to have a thermal expansion as large as -14 ppm/°C at low temperatures (60-110 K), becoming positive above 1100 K.[4] Its negative thermal expansion is a consequence of a traverse vibration of fluorine atoms that pull the
scandium atoms closer together (see figure).[5]
The fluorine atoms in scandium fluoride vibrate in a transverse direction to the scandium atoms to pull them together.
(Drawn using Inkscape.)
We can produce other NTE materials if we expand our purview to
metamaterials, where we can assemble structures from materials with different positive thermal expansions that work against each other to give a negative thermal expansion. That's the approach taken by
mechanical engineers at the
Massachusetts Institute of Technology, the
University of Southern California, the
University of California at Los Angeles, and
Lawrence Livermore National Laboratory, who used
projection microstereolithography to fabricate lightweight multimaterial lattices with significant negative thermal expansion in three directions over a temperature range of 170 °C.[6-9]
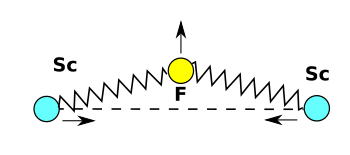
The research team showed how it's possible to produce NTE using connected structures of materials with different thermal expansions. In their
experiments, the materials were a
stiff copper-containing material of low thermal expansion, and an
elastic polymer of larger thermal expansion (see figure).[8]
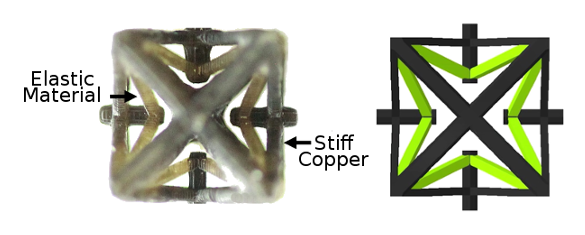
A negative thermal expansion metamaterial. The similarity between its action and the NTE principle of scandium fluoride can be seen. (Still images from an MIT YouTube Video.[9]
Says
Nicholas X. Fang, an
associate professor of
mechanical engineering at MIT,
"If we have proper placement of these beams and lattices, then even if every individual component expands, because of the way they pull each other, the overall lattice could actually shrink... once we increase the temperature, they interact with each other and pull inward, so the overall structure's volume decreases."[8]
The research team fabricated these metamaterial structures using a
3D printing method capable of printing two different materials. All
structural members were printed from the same polymer, but the polymer of some of the rods contained copper
nanoparticles that decreased the thermal expansion of those portions.[7] The structures were 5
millimeters wide, about the size of a
sugar cube, and the loading of the copper nanoparticles was chosen from the range 2%, 5%, and 10%.[7]
These structures achieved a volume reduction upon heating of up to 1% for the 10% copper specimens.[7] The size change was three times greater for 10% copper than for 2% copper, and the NTE was observed up to about 540
°F.[7] While the observed NTE is small, it does indicate that a material with a
zero thermal expansion can be made. Such materials would be useful for making
printed circuit boards.[8] This research was supported by the
Defense Advanced Research Projects Agency.[8]
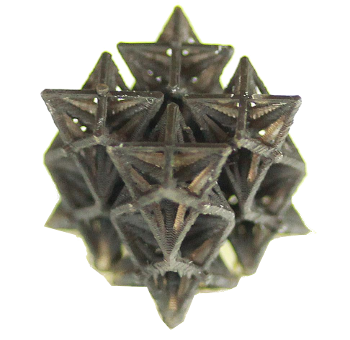
Three-dimensional metamaterial with a negative thermal expansion.
This structure was printed stereolithographically.
(MIT image by Qiming Wang.)
![]()
References:
- Douglas Wetmore Judson, "Method of making a connection," US Patent No. 3,396,460, August 13, 1968.
- Jason N. Hancock, Chandra Turpen, Zack Schlesinger, Glen R. Kowach and Arthur P. Ramirez, "Unusual Low-Energy Phonon Dynamics in the Negative Thermal Expansion Compound ZrW2O8," Phys. Rev. Lett., vol. 93, no. 22 (November 22, 2004), Document No. 225501.
- David Lindley, "Bake, Shake, and Shrink," Physical Review Focus, vol. 14, no. 21 (November 22, 2004).
- Benjamin K. Greve, Kenneth L. Martin, Peter L. Lee, Peter J. Chupas, Karena W. Chapman and Angus P. Wilkinson, "Pronounced Negative Thermal Expansion from a Simple Structure: Cubic ScF3," J. Am. Chem. Soc., vol. 132, no. 44 (November 10, 2010), pp 15496-15498.
- Chen W. Li, Xiaoli Tang, J. A. Muñoz, J. B. Keith, S. J. Tracy, D. L. Abernathy, and B. Fultz, "Structural Relationship between Negative Thermal Expansion and Quartic Anharmonicity of Cubic ScF3," Phys. Rev. Lett., vol. 107, no. 19 (November 4, 2011), Document No. 195504.
- Qiming Wang, Julie A. Jackson, Qi Ge, Jonathan B. Hopkins, Christopher M. Spadaccini, and Nicholas X. Fang, "Lightweight Mechanical Metamaterials with Tunable Negative Thermal Expansion," Phys. Rev. Lett. vol. 117, Article No. 175901, October 21, 2016, DOI:https://doi.org/10.1103/PhysRevLett.117.175901.
- Katherine Wright, "Focus: 3D Structure Shrinks When Heated," Physics, vol. 9, no. 121 (October 21, 2016).
- Jennifer Chu, "3-D-printed structures shrink when heated," MIT Press Release, October 25, 2016.
- Heat-induced shrinkage, Massachusetts Institute of Technology YouTube Video,October 25, 2016.
Permanent Link to this article
Linked Keywords: Electronics; toolbox; heat-shrink tubing; technician; cable; father; sin; strain-relief; electrical connector; invention; invented; thermoplastic; polytetrafluoroethylene; PTFE; polyvinyl chloride; PVC; polyolefin; tube; molecule; cross-link; electron; radiation; diameter; pressure; crystalline melting point; quenching; quench; room temperature; heat gun; hot air gun; polystyrene; Shrinky Dinks; craft; toy; butterfly; Wikimedia Commons; temperature; material; irreversible process; reversible process; thermal expansion; negative thermal expansion; linearity; linear; thermal expansion coefficient; room temperature; hydrogen bond; hydrogen-bonded; liquid; ethanol; carbon steel; water; soda-lime glass; mercury; aluminium oxide; alumina; aluminum; tungsten; stainless steel; borosilicate glass; copper; Invar; nickel; diamond; concrete; fused quartz; atom"; atomic; vibration; vibrational; energy; chemical bond; bonding force; crystal; molecule; freezing; electronic circuitry; silicon; kelvin; cubic zirconium tungstate; absolute zero; hafnium; zirconium; molybdenum; tungsten; analogy; analog; scandium fluoride; fluorine; scandium; molecular vibration; Inkscape; metamaterial; mechanical engineering; mechanical engineer; Massachusetts Institute of Technology; University of Southern California; University of California at Los Angeles; Lawrence Livermore National Laboratory; projector; projection; stereolithography; microstereolithography; experiment; stiffness; stiff; elasticity; elastic; metamaterial; YouTube; Nicholas X. Fang; associate professor; mechanical engineering at MIT; beam; latticework; lattice; volume; 3D printing; structural element; structural member; nanoparticle; millimeter; sugar cube; Fahrenheit; °F; zero thermal expansion; printed circuit board; Defense Advanced Research Projects Agency; Douglas Wetmore Judson, "Method of making a connection," US Patent No. 3,396,460, August 13, 1968.