Saturn and Metallic Hydrogen
July 27, 2015
The
periodic table is a good guide to
estimating the behavior of
elements. Elements in a
column have similar properties, since they have the same configuration of
valence electrons. It's not surprising that
aluminum,
gallium, and
indium are all
metals; that
carbon,
silicon, and
germanium are
semiconductors; and that
helium,
neon,
argon,
krypton, and
xenon are all
noble gases.
The elements of the first column of the periodic table are known as the
alkali metals, principally because they form
strong bases; e.g.,
sodium hydroxide, NaOH. That's how they're characterized by
chemists, but
physicists are more interested in their
electrical properties; so, in those circles, they're known as "simple metals." These metals are easy to understand, since they have just a single valence electron.
Gold and
silver are also classed as simple metals.
Scientists, no matter what their specialty, are all familiar with the periodic table of the elements. Most of the higher atomic number elements are known to just chemists and materials scientists. I've personally done experiments with 76 of the elements. (A composite of several Wikimedia Commons images.)
The alkali elements include both
hydrogen and
francium. Hydrogen is a
gas, and francium is a
radioactive element that's too unstable to exist as an observable
solid. All the solid alkali elements
crystallize in the
body-centered cubic (BCC)
crystal form (see figure). Francium is
theoretically predicted to also have this crystal structure. As metals, all the observed alkali elements have high
electrical conductivity and low
resistivity, as shown in the following table.[1]
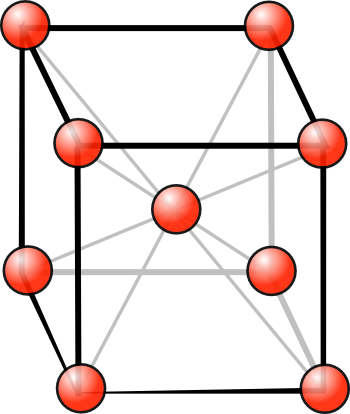
Arrangement of atoms in the body-centered cubic (BCC) crystal structure.
The BCC lattice can be pictured as two interpenetrating simple cubic lattices.
(Illustration by the author, rendered using Inkscape.)
Since it's often possible to crystallize gases with application of
high pressure and
low temperature, would hydrogen behave as a metal when crystallized? This question was considered by
Eugene Wigner, and his
colleague,
Hillard Bell Huntington, in 1935.[2] Their conclusion was that BCC hydrogen would be a metal, but only at a "
density many times higher than that of the ordinary,
molecular lattice of solid hydrogen."[2]
The
pressure at the
center of the Earth, about 365
GPa, can nearly be created in the
laboratory with an apparatus known as the
diamond anvil cell. Momentary high pressures around 100,000 GPa can be created in controlled
explosions. So, how high a pressure is needed to produce
metallic hydrogen? Wigner and Huntington
predicted just 25 GPa, but this estimate was found to be too low. Later estimates placed the pressure possibly within reach of a laboratory experiment, a few hundred GPa, provided that the
temperature was low enough.
While metallic hydrogen is unlikely to exist at the center of the Earth, the internal pressure of the
gas giant planets is much higher. It's
conjectured that a large
volume of the interiors of these planets is metallic hydrogen. The gas giants,
Jupiter and
Saturn are composed primarily of hydrogen, with some helium and other elements. The other gas giants,
Uranus and
Neptune, have less hydrogen and helium and more of the heavier elements. They are known also as
ice giants.
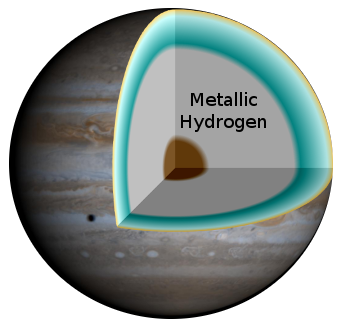
A Schematic diagram of the supposed interior of the gas giant, Jupiter. An intermediate region of metallic hydrogen is bracketed by an exterior region of mostly molecular hydrogen and an interior solid region of other elements.
(Labeled version of a NASA illustration by R.J. Hall, via Wikimedia Commons.)
Although Jupiter and Saturn are
compositionally similar, there's always been a discrepancy in
models that describe their
physical state. Planets cool as they age, but Saturn is warmer than it should be.
Computer models predict Jupiter's age correctly at about 4.5 billion years, but the prediction for Saturn is only 2.5 billion years. The transition point of solid, molecular hydrogen to metallic hydrogen is an important part of these models, but it's never been
experimentally determined.
Scientists at
Sandia National Laboratories (Albuquerque, New Mexico) and the
University of Rostock (Rostock, Germany) have just observed the
insulator-to-metal transition in a dense liquid of the hydrogen
isotope,
deuterium.[3-4] This measurement refines the conditions for which hydrogen and helium become
immiscibile, an important factor in models of the internal structure of these planets.[3-4] This hydrogen transition had never been physically observed.[4]

The planet, Saturn, as imaged by the Voyager 2 spacecraft on August 4, 1981.
Also imaged are three of its satellites, Tethys, Dione, and Rhea, with the shadow of Tethys appearing prominently on Saturn.
(NASA/JPL image via Wikimedia Commons.)
The
experiment was done using Sandia's
Z machine, an
X-ray generator that produces an intense
electromagnetic pulse as part of its operation. The
magnetic field associated with the
sub-microsecond pulse was used to shocklessly squeeze deuterium at low temperature. This experiment overcame the side-effect of
shock compression experiments that the hydrogen temperature is also increased.[4]
Says Marcus Knudson, one of the two lead
authors of the
paper in
Science[3] presenting this experiment,
"We started at 20 degrees Kelvin, where hydrogen is a liquid, and sent a few hundred kilobar shock - a tiny flyer plate pushed by Z's magnetic field into the hydrogen - to warm the liquid... Then we used Z's magnetic field to further compress the hydrogen shocklessly, which kept it right above the liquid-solid line at about 1,000 degrees K."[4]
When the
liquid hydrogen was compressed to more than twelve times its initial density, at a pressure of three
megabars (300 GPa)
instruments detected that the hydrogen was in an atomic, rather than a molecular, state.[4] Since the electromagnetic pulse prohibited electrical measurements, the detection of metallic hydrogen was done
optically. A 45%
reflectivity was detected, and this could only have been achieved by a metal.[4]
In the planetary model of Saturn, metallic hydrogen mixed with helium at Saturn's temperature will release helium
rain, an
energy distribution mechanism that alters the planet's
evolution. The helium rain would keep Saturn warmer than expected.[4] The Z-machine is funded by the
US National Nuclear Security Administration.[4]
References:
- Electrical resistivities of the elements (data page), Wikipedia.
- E. Wigner and H. B. Huntington, "On the Possibility of a Metallic Modification of Hydrogen," J. Chem. Phys., vol. 3, no. 12 (December 1, 1935), p. 764ff..
- M. D. Knudson, M. P. Desjarlais, A. Becker, R. W. Lemke, K. R. Cochrane, M. E. Savage, D. E. Bliss, T. R. Mattsson, and R. Redmer, "Direct observation of an abrupt insulator-to-metal transition in dense liquid deuterium," Science, vol. 348 no. 6242 (June 26, 2015), pp. 1455-1460.
- Sandia's Z machine helps solve Saturn's 2-billion-year age gap, Sandia National Laboratory Press Releases, June 26, 2015.
Permanent Link to this article
Linked Keywords: Periodic table; approximation; estimating; chemical element; group; column; >valence electron; aluminum; gallium; indium; metal; carbon; silicon; germanium; semiconductor; helium; neon; argon; krypton; xenon; noble gas; alkali metal; strong base; sodium hydroxide; chemist; physicist; electricity; electrical; gold; silver; scientist; branches of science; specialty; atomic number; chemist; materials scientist; Wikimedia Commons; hydrogen; francium; gas; radioactive decay; solid; crystallization; crystallize; cubic crystal system; body-centered cubic; crystal; theory; theoretical; electrical conductivity; electrical resistivity; Hydrogen (H); Lithium (LI); Sodium (Na); Potassium (K); Rubidium (Rb); Cesium (Cs); Francium (Fr); simple cubic; Inkscape; high pressure; cryogenics; low temperature; Eugene Wigner; colleague; Hillard Bell Huntington; density; molecule; molecular; pressure; center of the Earth; pascal; GPa; laboratory; diamond anvil cell; explosion; metallic hydrogen; prediction; predicted; temperature; gas giant planet; conjecture; conjectured; volume; Jupiter; Saturn; Uranus; Neptune; ice giant; Schematic diagram; analytical chemistry; composition; mathematical model; physics; physical; computer model; experiment; experimentally; Sandia National Laboratories (Albuquerque, New Mexico); University of Rostock (Rostock, Germany); insulator; isotope; deuterium; miscibility; immiscibile; planet; Voyager 2 spacecraft; moons of Saturn; satellite; Tethys; Dione; Rhea; shadow; Wikimedia Commons; Z Pulsed Power Facility; Z machine; X-ray generator; electromagnetic pulse; magnetic field; sub-microsecond; pulse; shock compression; author; academic publishing; paper; Science; kelvin; kilobar; liquid hydrogen; megabar; instrument; optics; optically; reflectance; reflectivity; rain; energy; evolution; US National Nuclear Security Administration.