Impact Diamonds
January 26, 2015
Diamonds Are Forever is a 1971
James Bond film staring
Sean Connery.[1] The title comes from the popular notion that
diamonds are indestructible. Diamonds, like other
carbon allotropes, will actually
burn. As I outlined in a
previous article (Making Diamonds, December 16, 2013), the Gibbs free energy shows that oxidation is favored even at
room temperature; viz.,[2]
C(Diamond) + O2(gas) -> CO2(gas)
ΔGf(Diamond) = 0.693 kcal/mole
ΔGf(Oxygen) = 0 kcal/mole
ΔGf(Carbon Dioxide) = -94.258 kcal/mole
ΔG(Reaction) = -94.951 kcal/mole
The negative sign indicates a favorable
reaction. However, an
activation energy must be overcome before the reaction initiates. For this reason, diamond will only ignite at a temperature of 850-1,000 °C in
air, or 720-800 °C in pure
oxygen.
Theory is one thing, but
scientists are skeptical people, and they require
proof in the form of an
experiment. In 1772,
Antoine Lavoisier used a lens to focus the
Sun's rays onto a diamond in an oxygen atmosphere to heat it sufficiently to produce carbon dioxide.
Diamond is the stable allotrope of carbon only under high
pressure, as its
Phase Diagram shows (see figure). Fortunately for
jewelers, once diamond is formed it will exist
metastably at room temperature. Natural diamonds form from
carbonaceous minerals at extreme depths (about a hundred
miles) within
Earth's mantle where such high pressures exist. This process was completed only after a considerable fraction of the age of the Earth had passed. The diamonds thus formed were transported to the surface by
volcanic eruptions in which they are embedded in igneous rock, such as kimberlite.
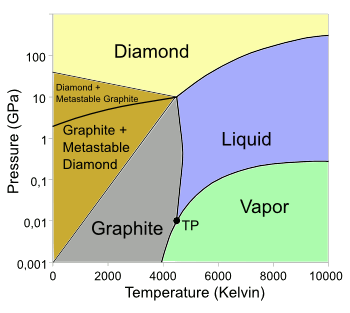
The carbon phase diagram.
The triple point (TP) is around 4600 K and 10.8 MPa.
(Wikimedia Commons image, from data in ref. 3, modified using Inkscape.)[3)]
Geologic pressure can be obtained in another, spectacular way by
meteor impact. Meteor impact happens at about 17
km/s, but impacts large enough to leave a significant
crater (a kilometer in diameter) happen only every five thousand years. The
energy involved in such impacts is about 40
megatons, with
extinction level 50
gigaton events happening a few times every million years.

There goes the neighborhood!
An artist's impression of the Cretaceous-Tertiary (K-T) meteor impact event, now known as the Cretaceous-Paleogene (K-Pg) impact event.
This meteor impact, 65 million years ago, is thought to have been responsible for dinosaur extinction.
(Image: Don Davis/NASA, via Wikimedia Commons.)
Many meteors contain
graphitic carbon, and the class of meteors called
carbonaceous chondrites even have carbon in their names. Impact of these meteors creates small diamonds from the simultaneous heat and pressure, and these diamonds were identified as a type with a
crystal structure similar to the original
graphite. This mineral
phase, discovered in 1967 in the remains of the
Canyon Diablo meteorite, was named
lonsdaleite.

This "lonsdaleite" grain from the Canyon Diablo meteorite looks more like coal than diamond.
The scale bar is one millimeter.
(Photograph: Arizona State University/Laurence Garvie, cropped and annotated.)[5)]
An international team of scientists from the
Hungarian Academy of Sciences (Budapest, Hungary),
Arizona State University (Tempe, Arizona), and the
University of Bayreuth (Bayreuth, Germany) decided to look more closely at the crystal structure of lonsdaleite. The motivation for this was the inability to
synthesize lonsdaleite as a separate, pure
material. In a recent
paper in
Nature Communications, they provide evidence that lonsdaleite is actually a structurally disordered form of ordinary diamond.[4-5]
Not only has the presence of lonsdaleite been used as an indicator of meteor impact, it's been conjectured that lonsdaleite might have
mechanical properties, such as high
strength, superior to ordinary diamond. The lure of such high strength has inspired efforts for its synthesis, and the failure of such synthesis has puzzled scientists for decades.[5]
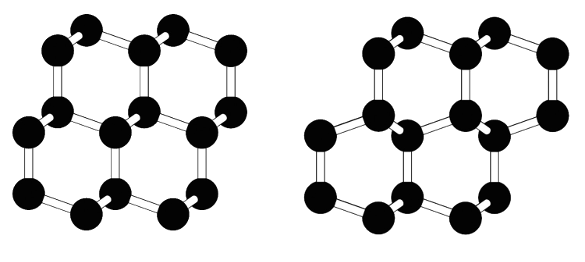
The crystal structure of diamond (left), and the presumed structure of lonsdaleite (right). The carbon atoms are tetrahedrally-bonded in each, but the overall structure shows a subtle difference between alternate layers of carbon atoms. (Arizona State University image by Péter Németh.)[5)]
The
research team attempted synthesis of lonsdaleite, and it also examined Canyon Diablo specimens of the mineral using advanced
electron microscope techniques. They discovered diamond with extensive {113}
twins and {111}
stacking faults, and they identified these novel defects also in their synthetic samples.[4-5] These defects cause the same
X-ray and
electron reflections reported for lonsdaleite.[4] Their conclusion is that lonsdaleite is just the regular
cubic form of diamond in which such defects have been generated by
shock or pressure.[5]
Says Péter Németh of the Hungarian Academy of Sciences, a former visiting scientist at Arizona State University, and
coauthor of the study, "So-called lonsdaleite is actually the long-familiar cubic form of diamond, but it's full of defects."[5] Still, "lonsdaleite" might show some interesting mechanical properties, including exceptional
hardness.[5]
References:
- Diamonds Are Forever (1971, Guy Hamilton, Director) on the Internet Movie Database.
- Free energy data from L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- J.M. Zazula, "On Graphite Transformations at High Temperature and Pressure Induced by Absorption of the LHC Beam," LHC Project Note 78, CERN, January 18, 1997 (PDF File).
- Péter Németh, Laurence A. J. Garvie, Toshihiro Aoki, Natalia Dubrovinskaia, Leonid Dubrovinsky, and Peter R. Buseck, "Lonsdaleite is faulted and twinned cubic diamond and does not exist as a discrete material," Nature Communications, vol. 5, Article No. 5447 (November 20, 2014), doi:10.1038/ncomms6447.
- Asteroid impacts on Earth make structurally bizarre diamonds, say ASU scientists, Arizona State University Press Release, November 20, 2014.
Permanent Link to this article
Linked Keywords: Diamonds Are Forever; James Bond; film; Sean Connery; diamond; carbon allotrope; combustion; burn; room temperature; kilocalorie per mole; kcal/mole; oxygen; carbon dioxide; ngative number; negative sign; chemical reaction; activation energy; material properties of diamond; ignition temperature; celsius; °C; atmosphere of Earth; air; oxygen; scientific theory; scientist; proof; experiment; Antoine Lavoisier; lens; focus; Sun; pressure; phase diagram; jeweler; metastable; carbonaceous; mineral; mile; Earth's mantle; age of the Earth; volcanic eruption; igneous rock; kimberlite; triple point; Wikimedia Commons; Inkscape; geology; geologic; impact event; meteor impact; kilometers per second; km/s; crater; energy; TNT equivalent; megaton; extinction event; gigaton; artist's impression; Cretaceous-Tertiary (K-T) meteor impact event; Cretaceous-Paleogene (K-Pg) impact event; dinosaur; Don Davis; NASA; graphitic; carbonaceous chondrite; crystal structure; graphite; phase; Canyon Diablo meteorite; lonsdaleite; coal; linear scale; scale bar; millimeter; Laurence Garvie; Hungarian Academy of Sciences (Budapest, Hungary); Arizona State University (Tempe, Arizona); University of Bayreuth (Bayreuth, Germany); chemical synthesis; synthesize; material; scientific literature; paper; Nature Communications; mechanical property; strength; crystal structure; atom; tetrahedral symmetry; tetrahedrally bonded; Péter Németh; research; electron microscope; crystal twinning; stacking fault; X-ray; electron; Bragg's law; reflection; diamond cubic; cubic form of diamond; shock; coauthor; hardness; Diamonds Are Forever (1971, Guy Hamilton, Director) on the Internet Movie Database.