Persistent Photoconductivity
November 27, 2013
Selenium would have been an uncommon
material were it not for two
electrical properties discovered in the very early days of
electronics. The most useful of these is its ability to act as a type of
rectifier called a
metal rectifier. This rectifier action (changing
alternating current to
direct current) was discovered by
Charles Fritts, circa 1886, but such rectifiers were only made practical in the
1930s.
The selenium rectifier elements, looking like the fins of today's
semiconductor heat sinks (see photograph), were
aluminum or
steel plates, coated with a thin layer of
bismuth or
nickel, and then coated with a thick (~0.0025
inch) layer of selenium.
Heat treatment ensured conversion of the selenium to its gray,
hexagonal phase.
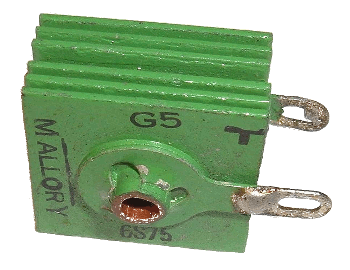
A selenium rectifier with inch square plates. Selenium rectifiers were common when I first started experimenting with electronics in the early 1960s.
(Photograph by Arnold Reinhold, viaWikimedia Commons.)
Each selenium element had a
breakdown voltage of about twenty-five
volts, but putting many in series gave a high voltage rectifier. Each selenium element had a
voltage drop of about a volt, compared with about 0.7 volts for
silicon rectifiers. Selenium rectifiers were useful for high current rectification, as for
automotive battery chargers; but the voltage drop of multiple
series connections, combined with the high current, resulted in a hot rectifier (up to 130°
C) and a
service life of about 50,000 hours.
The other useful property of selenium is its
photoconductivity, which was discovered in 1873 by the
English electrical engineer,
Willoughby Smith. As often happens in
science, this discovery was
accidental. Smith was doing electrical measurements on
submarine telegraph cables, and he found that the selenium resistance reference material he was using became more
conductive upon exposure to
light.[1]
Complementary to selenium's photoconductivity was the 1877 discovery of the
photovoltaic effect in selenium by W.G. Adams and R.E. Day.[2]
Charles Fritts used this effect to make a selenium-
gold solar cell in 1883.[3] This solar cell had less than 1%
efficiency, but it was an innovation, nonetheless. The best photovoltaic cells of today have better than 35% efficiency, but residential panels have
far less than that.
Selenium's photoconductivity was crucial to the 1938 invention of
Xerography by
Chester Carlson. Carlson found that a plate coated with "
cuprous oxide or the metallic variety of selenium," would lose
charge in the areas exposed to light. Thus, an image of a printed page would still have charge at the black letters, and none from the white background sheet. A fine, black powder,
sticking to the charged surface, would form a copy of the image.[4]
The photoconductivity of selenium was a surprise, and you would think that few such surprises would still exist for simple materials.
Strontium titanate (SrTiO
3) was first
synthesized by Leon Merker at the
National Lead Company, and a process for its
synthesis was patented by Merker in 1956.[5] Now, more than fifty years later,
physicists at
Washington State University (Pullman, Washington) have discovered persistent photoconductivity in strontium titanate.[6-8]
Persistent photoconductivity means that shining a light on the material increases the conductivity not just for the period of light exposure, but for a significant time thereafter. In the case of strontium titanate, this time period is many days, which means that it would be possible to make an
optical memory device using this effect.[7]
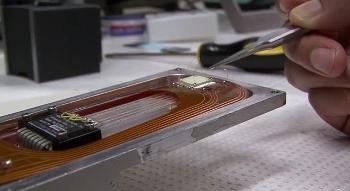
Mounting a strontium titanate specimen (white square) for measurement.
(Still image from a YouTube video.[8]
Washington State University
doctoral student,
Marianne Tarun, discovered the effect. A research team comprised of Tarun, fellow student,
Farida Selim, now at
Bowling Green State University, and
professor Matthew McCluskey, found through
experiments that the effect was not from some
dopant, but it was an effect of light on
pure strontium titanate.[7] Said Tarun, "It came by accident... It's not something we expected. That makes it very exciting to share."[7]
Exposing strontium titanate at
room temperature to
light energy at its
intrinsic bandgap of 2.9
eV or higher increases the
free-electron concentration by more than two
orders of magnitude.[6] The conductivity jumps by a factor of 400, albeit from a low initial level.[7]
The higher conductivity remains for days with negligible decay, and the authors speculate that the persistent photoconductivity is due to excitation of
electrons from a
titanium vacancy defect to the
conduction band, with subsequent electron recapture by the vacancy having a very low
probability.[6]
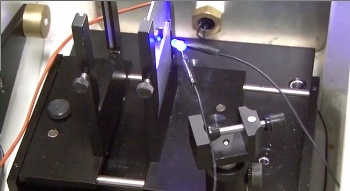
Measuring photoconductivity of a strontium titanate specimen.
(Still image from a YouTube video.[8]
Says paper co-author, Matthew McCluskey, alluding to the possibility of using strontium titanate as a
holographic memory,
“The discovery of this effect at room temperature opens up new possibilities for practical devices. In standard computer memory, information is stored on the surface of a computer chip or hard drive. A device using persistent photoconductivity, however, could store information throughout the entire volume of a crystal.”[7]
The Washington State University research was funded by the
National Science Foundation.[7]
References:
- "Effect of Light on Selenium during the passage of an Electric Current" (W. Smith), Nature, vol. 7, no. 173 (February 20, 1873), pp. 303-303.
- W. G. Adams and R. E. Day, "The Action of Light on Selenium," Proc. R. Soc. Lond., vol. 25 (January 1, 1876), pp. 113-117, doi:10.1098/rspl.1876.0024 (PDF File).
- C. E. Fritts, "On a new form of selenium cell, and some electrical discoveries made by its use," American Journal of Science, vol. 26, series 3 (December, 1883), pp. 465-472.
- Chester F Carlson, "Electrophotography," US Patent No. 2,297,691, October 6, 1942.
- Leon Merker, "Refractive material," US Patent No. 2,764,490, September 25, 1956.
- Farida A. Selim and Matthew D. McCluskey, "Persistent Photoconductivity in Strontium Titanate," Physical Review Letters, vol. 111, no. 18 (November 1, 2013), Document No. 187403 [5 pages].
- Accidental discovery dramatically improves conductivity, Washington State University Press Release, November 14, 2013.
- Washington State University, "Accidental Discovery Dramatically Improves Electrical Conductivity," YouTube Video, November 15, 2013.
Permanent Link to this article
Linked Keywords: Selenium; material; electricity; electrical; electronics; selenium rectifier; metal rectifier; alternating current; direct current; Charles Fritts; 1930s; semiconductor; heat sink; aluminum; steel; bismuth; nickel; inch; heat treating; Heat treatment; hexagonal crystal system; hexagonal phase; Wikimedia Commons; breakdown voltage; volt; voltage drop; diode; silicon rectifier; automobile; automotive; battery charger; series circuit; series connection; Celsius; C; service life; photoconductivity; English; electrical engineer; Willoughby Smith; science; serendipity; accidental; submarine telegraph cable; electrical conductivity; conductive; light; photovoltaic effect; Charles Fritts; gold; solar cell; efficiency; Xerography; Chester Carlson; cuprous oxide; electric charge; Coulomb's law; electrostatic attraction; strontium titanate; chemical synthesis; NL Industries; National Lead Company; physicist; Washington State University (Pullman, Washington); optics; optical; computer memory device; YouTube video; Doctor of Philosophy; doctoral student; >Marianne Tarun; Farida Selim; Bowling Green State University; professor; Matthew McCluskey; experiment; dopant; intrinsic semiconductor; room temperature; radiant energy; light energy; electronvolt; eV; free-electron; orders of magnitude; electron; titanium; vacancy defect; conduction band; probability; holographic data storage; holographic memory; National Science Foundation; Chester F Carlson, "Electrophotography," US Patent No. 2,297,691, October 6, 1942; Leon Merker, "Refractive material," US Patent No. 2,764,490, September 25, 1956.