Silver Ink
January 19, 2012
When I was a
graduate student, I needed a very small amount of
silver, just a few
grams, as an
alloy addition. Our supplies cabinet was bursting with bottles of
metal powders, mostly
iron and its neighbors in the
periodic table, but there was no silver. I asked my
thesis advisor if we could order some, but the minimum order was more than ten times what I needed.
Materials scientists come from varied backgrounds. I came from
physics, but my advisor studied
chemistry as an
undergraduate. He sent me to a friend in the chemistry department to fetch two chemicals that are commonly used in undergraduate teaching
laboratories. These are
silver nitrate (AgNO
3) and
aqueous ammonia (
ammonium hydroxide, NH
4OH). He mixed the two, and some others that I can't remember, and magically precipitated pure silver from the solution.
The overall reaction produced
ammonium nitrate (NH
4NO
3), which remained in solution, and silver. Reactions in which silver is produced are used also as an
analytical test.
Tollen's reagent, which is based on silver nitrate, along with ammonium hydroxide and other chemicals, is used as a test for a
carbonyl group chemical. When an
aldehyde is mixed with this reagent, silver will precipitate. Variants of this reaction are used to
silver mirrors.
Scientists from the
Department of Materials Science and Engineering and the
Frederick Seitz Materials Research Laboratory at the
University of Illinois at Urbana-Champaign have modified the Tollen's reaction as a way to write silver conductors, and they have published their research in a recent issue of the
Journal of the American Chemical Society.[1-2] Their objective was the production of a silver
ink that can be processed at
temperatures low enough to write conductors on inexpensive and flexible
plastic, or even
paper.

Silver ink airbrushed onto a plastic film to make a flexible silver electrode.
(Photo by S. Brett Walker. Used with permission.)
Their silver ink is actually a
precursor for silver, and not an ink in the conventional sense. Inks are
colloids of
pigment particles in a
fluid. The limitation of colloid ink is that the writing nozzle needs to be larger in diameter than the largest particle in solution. This places limits on the smallest linewidth that can be written with an ink, which is generally several tens of
micrometers.[1]
Other silver precursor inks have been demonstrated to produce conductive traces with the same conductivity as bulk silver, but these require a decomposition reaction near 150 °C.[1] This is too high for most
polymeric substrates, but not too high for
polyimide. The University of Illinois Tollen's reaction is based on
silver acetate, rather than silver nitrate. Their resulting ink has 22 wt-% silver.[1]
The reactive silver ink can be printed through nozzles of just 100
nm diameter, and it can be
inkjet-printed and
airbrush-sprayed if 10% by volume
2,3-butanediol is added to change the
viscosity and maintain moisture content. Since the ink is a precursor, silver is formed only when the fluid evaporates and the material is heated. The dried solution contains
diaminesilver (Ag(NH
3)
2CH
3CO
2), which decomposes to pure silver and
volatile chemicals that are released at heating.[1]
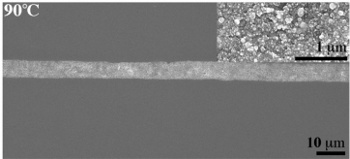
Microstructure of the silver ink annealed at 90 °
(Photomicrograph courtesy of S. Brett Walker. Used with permission.)
Annealing the printed traces at 90 °
C produces silver conductors with the same conductivity as bulk silver (>6.3 x 10
5 siemens/
cm).[1] This is the highest reported conductivity in a printed ink. Says paper coauthor, S. Brett Walker,[2]
"For printed electronics applications, you need to be able to store the ink for several months because silver is expensive... Since silver particles don't actually form until the ink exits the nozzle and the ammonia evaporates, our ink remains stable for very long periods. For fine-scale nozzle printing, that's a rarity... We are now focused on patterning large-area transparent conductive surfaces using this reactive ink."
This research was supported by the
National Science Foundation and the
U.S. Department of Energy.[2]
References:
- S. Brett Walker and Jennifer A. Lewis, "Reactive Silver Inks for Patterning High-Conductivity Features at Mild Temperatures," J. Am. Chem. Soc., (Article ASAP, January 5, 2012), DOI: 10.1021/ja209267c.
- Liz Ahlberg, "Particle-free silver ink prints small, high-performance electronics," University of Illinois Press Release, January 12, 2012.
Permanent Link to this article
Linked Keywords: Graduate student; silver; gram; alloy; metal; iron; periodic table; thesis advisor; materials scientist; physics; chemistry; undergraduate; laboratory; silver nitrate; aqueous; ammonia; ammonium hydroxide; ammonium nitrate; analytical test; Tollen's reagent; carbonyl group; aldehyde; silver mirroring; scientists; Department of Materials Science and Engineering; Frederick Seitz Materials Research Laboratory; University of Illinois at Urbana-Champaign; Journal of the American Chemical Society; ink; temperature; plastic; paper; precursor; colloid; pigment; fluid; micrometer; polymer; polyimide; silver acetate; nanometer; nm; inkjet; airbrush; 2,3-butanediol; viscosity; diaminesilver; volatile; annealing; celsius; C; siemens; centimeter; cm; National Science Foundation; U.S. Department of Energy.