Carbon Voltaic Energy
June 27, 2012
We live in a world of constant
flow, much of which is useful.
Hydroelectricity is actually a form of
solar energy. It's created from the flow of
water, and it presently provides a little more than six percent of the
electricity in the
United States. The flow of
ocean water in the tides is another source of electrical energy, as I described in a
recent article (Tidal Forces, June 20, 2012). You could claim a small fraction of that as being solar energy, but that flow is mostly the work of the
Moon.
Flow on smaller scales yields electrical power in some unusual ways. Rubbing certain materials together generates
static electricity by the
triboelectric effect, and this trick will work also with fluid flow. In one demonstration, a voltage of 300
millivolts (mV) was obtained with a flow of pure water at 45 cm
3/sec in a
millimeter diameter
pipe.[1] This is also a cautionary tale of what materials you can use in
gasoline filling pipes.
Carbon nanotubes will generate a few millivolts when water flows over them because of coupling between
ions in the water and
charge carriers in the nanotubes. Since
graphene is the wonder material of the new
millennium, it's only reasonable that
scientists would try it in similar triboelectric experiments. Engineers at
Rensselaer Polytechnic Institute (Troy, New York) and
Rice University (Houston, Texas) found that adding ions, in the form of
hydrochloric acid (HCl), to the water gives an
order of magnitude higher voltage when the flow is over a graphene film (see figure).
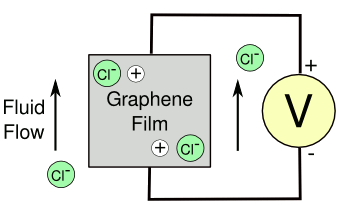
Flow of chlorine ions generating a current in a graphene film. The moving chlorine ions might drag associated electron holes in the graphene sheet.
(Drawing rendered by the author using Inkscape)
The RPI-Rice team was able to generate 85
nanowatts of
power for a 0.01
m/s flow of 0.6
M HCl over a 30 x 16
μm graphene film. This scales to a phenomenal 175 W/m
2. Computer simulations indicate that the currents in the graphene are a result of the flow of chlorine ions across the film.
When doing
experiments, I often used common
household materials that I had on hand if they served a purpose. I'm certain that many experiments of today are held together by
duct tape, just as in
Ernest Rutherford's time they were held together with
string and
sealing wax. When one of Rutherford's students needed a
metal tube for an experiment, Rutherford cut a piece from the
handlebar of an old
bicycle.[3]

When your funding is good, you reach for the Kapton tape (right), but most of the time, it's the duct tape (left).
(Photo by author, via Wikimedia Commons)
One common item found in all
laboratories is the
pencil. Although these were called "lead pencils" when I was young, the writing material is not
lead. It's a
composite material of
graphite powder in a
clay binder. It's
conductive, and traces on
paper are also conductive; and also
piezoresistive, as I demonstrated in a
previous article (Paper Accelerometer, March 10, 2011). Pencil lead is not exactly graphene, but it might share some of its properties.
That must be what some scientists were thinking when they made an energy cell from pencils in various metal
chloride solutions. These are claimed to be no ordinary type of
energy harvester, but a device that converts
ambient thermal energy into small amounts of electrical power. We're not talking
batteries, or
thermopower devices, but a fundamental way to convert the energy of a
constant temperature bath into electricity.[4]
Parallel pencil leads in three
molar potassium chloride (KCl),
sodium chloride (NaCl),
nickel(II) chloride (NiCl
2) and
copper(II) chloride (CuCl
2) solutions at room temperature produced 0.655, 1.023, 1.023 and 1.828 nanowatts, respectively. This was also the case for
graphene oxide and
graphene films. To squelch possible criticism of their methods, the authors state that in no case were any connecting wires in contact with the solutions, a condition that would give an
electrochemical voltage. As an understatement, the authors write that the mechanism is still unclear.[4]

Graphite thermal energy device built from pencils.
The green color of the solution indicates that it's a nickel(II) chloride solution.
(Via arXiv Preprint Server).[4)]
This work can be considered to be a follow-up to experiments published in March that showed this effect for graphene in a saturated CuCl
2 solution.[5] In those experiments, 0.35 volts were generated for twenty days, and there was a
positive correlation between the open-circuit voltage and the
temperature, and the open-circuit voltage and the
cation concentration. Both of these correlations would be obtained from such a supposed thermal energy harvester.[5]
The research team, composed of scientists from the
Hong Kong Polytechnic University,
Pacific Northwest National Laboratory, and
Nanjing University of Aeronautics and Astronautics, were able to light a
light-emitting diode using a
series combination of six of these devices.
Since the amount of graphene is small, the calculated
power density is about 70 KW/Kg, but this ignores the solution. The claimed power source is the thermal motion of ions in the solution, which have
speeds of hundreds of meters per second at room temperature, and energies of about 4 kJ⋅kg
-1⋅K
-1.
All this sounds too good to be true, so there's quite a bit of skepticism among scientists.[6] Unexpected results like these are interesting, because they can lead to new lines of scientific inquiry. At the same time, science says that
extraordinary claims require extraordinary evidence. Wanlin Guo, the graduate supervisor of one of the team members, Guoan Tai, while he was at Nanjing University, has never seen an output voltage greater than about 0.1 millivolts in his own experiments; then again, he did see a voltage.[6]
Zihan Xu, an author of both thermal device papers, says that he is "100% confident" that his experiments are true and that he can "repeat them anywhere and anytime."[6]
Nikhil Koratkar, an author of the study involving the generation of electricity from ions flowing past graphene, thinks that there might be something there.[6] In any case, the device is so simple to prepare that many confirmatory experiments will be attempted in various laboratories.[7] That's the true nature of science.
References:
- B. Raveloa, F. Duvala, S. Kanea and B. Nsomb, "Demonstration of the triboelectricity effect by the flow of liquid water in the insulating pipe," Journal of Electrostatics, vol. 69, no. 6 (December, 2011), pp. 473-478.
- Prashant Dhiman, Fazel Yavari, Xi Mi, Hemtej Gullapalli, Yunfeng Shi, Pulickel M. Ajayan and Nikhil Koratkar, "Harvesting Energy from Water Flow over Graphene," Nano Letters, vol. 11, no. 8 (August 10, 2011), pp. 3123-3127.
- Richard Reeves, "A Force of Nature: The Frontier Genius of Ernest Rutherford" (W. W. Norton, December 3, 2007, ISBN-13:978-0393057508), via Amazon.
- Zihan Xu, and Guo'an Tai, "Electricity generated from Ambient Heat by Pencils," arXiv Preprint Server, June 17, 2012.
- Zihan Xu, Guoan Tai, Yungang Zhou, Fei Gao, Kin Hung Wong, "Self-Charged Graphene Battery Harvests Electricity from Thermal Energy of the Environment," arXiv Preprint Server, March 12, 2012.
- Edwin Cartlidge, "Sparks fly over graphene energy device," Nature Blog, March 15, 2012.
- Since I'm a thermodynamicist, one experiment I would do is light an external LED while monitoring the device temperature in an insulated vessel, such as a Dewar flask. The device should cool, since conservation of energy is always supposed.
Permanent Link to this article
Linked Keywords: Fluid dynamics; flow; hydroelectricity; solar energy; water; electricity; United States; ocean; Moon; static electricity; triboelectric effect; millivolt; millimeter; pipe; gasoline; carbon nanotube; ion; charge carrier; graphene; millennium; scientist; Rensselaer Polytechnic Institute (Troy, New York); Rice University (Houston, Texas); hydrochloric acid; order of magnitude; chlorine; electric current; electron hole; Inkscape; nanowatt; power; meter per second; m/s; molar concentration; micrometer; μm; experiment; household; material; duct tape; Ernest Rutherford; twine; string; sealing wax; metal; tube; handlebar; bicycle; Kapton; duct tape; Wikimedia Commons; laboratory; pencil; lead; composite material; graphite; clay; binder; electrical conductivity; conductive; paper; piezoresistive; paper accelerometer; chloride; energy harvester; room temperature; ambient; thermal energy; battery; thermopower; thermal reservoir; constant temperature bath; molar; potassium chloride; sodium chloride; nickel(II) chloride; copper(II) chloride; graphene oxide; graphene; electrochemical; nickel(II) chloride; arXiv Preprint Server; positive correlation; temperature; cation; Hong Kong Polytechnic University; Pacific Northwest National Laboratory; Nanjing University of Aeronautics and Astronautics; light-emitting diode; series combination; power density; speed; extraordinary claims require extraordinary evidence; Nikhil Koratkar; thermodynamicist; thermal insulation; Dewar flask; conservation of energy.