| Tikalon Blog is now in archive mode.
An easily printed and saved version of this article, and a link
to a directory of all articles, can be found below: |
|
This article |
| Directory of all articles |
Scratching Diamond
December 6, 2010
It was a common movie cliché, testing whether the
diamond was real by scratching a mirror. It was also an example of the
Mohs scale of mineral hardness. This scratch test was more believable in the black and white movie era, before
cubic zirconia became commercially available. Cubic zirconia has a Mohs hardness of 8.5, and diamond has a Mohs hardness of 10. These will both scratch glass, which has a Mohs hardness of only 6 or 7. There are quite a few minerals that will scratch glass,[1] including
quartz, which even
looks somewhat like diamond. Only diamond will scratch diamond, as long as you're willing to ignore
borazon, a cubic form of
boron nitride, and possibly
beta carbon nitride. The Mohs Hardness Scale appears below.
| Mohs Hardness | Sclerometer Hardness | Mineral | Composition |
| 1 | 1 | Talc | Mg3Si4O10(OH)2 |
| 2 | 3 | Gypsum | CaSO4(H2O)2 |
| 3 | 9 | Calcite | CaCO3 |
| 4 | 21 | Fluorite | CaF2 |
| 5 | 48 | Apatite | Ca5(PO4)3(OH,Cl,F) |
| 6 | 72 | Orthoclase Feldspar | KAlSi3O8 |
| 7 | 100 | Quartz | SiO2 |
| 8 | 200 | Topaz | Al2SiO4(OH,F)2 |
| 9 | 400 | Corundum | Al2O3 |
| 10 | 1600 | Diamond | C |
The idea of scratch testing materials goes all the way back to
Theophrastus, whom I mentioned in a
previous article (Pyroelectric Energy Harvesting, October 15, 2010). In 314 BC, he wrote about minerals that iron could, or could not, scratch in his book, "On Stones."[2] Scratch tests and other tests of gems were noted by
Pliny the Elder in his
Naturalis Historia (c. 77). Pliny writes that diamond dust is used to cut and polish other gems and that diamond will scratch all other minerals.[3]

Portion of "On Stones" by Theophrastus concerning the fact that iron can shatter minerals that it can't scratch.[2]
You can think of many reasons why a mineral of a higher hardness could scratch one of a lower hardness, the most obvious of these being that the
chemical bonds that hold one together are stronger than the other. Also, you can think of reasons why imperfect
crystals of the same kind could scratch each other, since more perfect regions of one could occasionally attack less perfect regions of the other. But how can a perfectly crystalline diamond scratch another?
The reason is
anisotropy. The wear rate for diamond depends on which crystal face is exposed. Some
lattice planes are easier to polish than others. Although this anisotropy was not understood by scientists who study
tribology, craftsmen have made use of it since at least Pliny's time. The diamond polishing process employed for the last few hundred years involves pressing diamonds against a rotating iron wheel with fine diamond particles embedded in its surface. These wheels are rotated at about 30 meters per second to allow the craftsman holding the stone to get audible feedback as to when the diamond he's holding is at just the right angle.[4]
A research team at the
Fraunhofer Institute for Mechanics of Materials in
Freiburg, Germany, has just published a paper in
Nature Materials in which they use
molecular dynamics modeling to explain how diamonds can be machined.[5] They used
quantum mechanics to investigate bond breaking at the surface of diamond in calculations that involved 10,000
carbon atoms. What they found was a change in carbon
bond character from sp
3 to sp
2 that resulted in an
amorphous, glass-like, layer of carbon at the surface. The growth rate of this amorphous layer depended on surface orientation and sliding direction, in agreement with experimentally determined wear rates. Carbon is removed from the diamond surface by mechanical scraping; or by
oxidation of this layer by ambient
oxygen to form
carbon dioxide (see figure).[5]
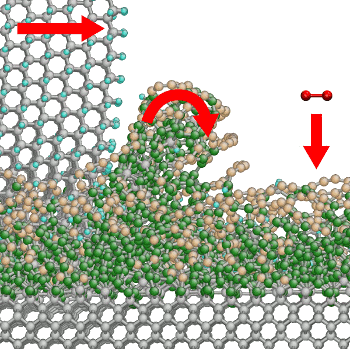
Material removal mechanism during diamond polishing
A sharp-edged diamond particle "peels off" a dust particle from the glass-like phase at the surface of the diamond as oxygen from the air reacts with the carbon at the surface to form carbon dioxide.
(Fraunhofer Institute Illustration)
Diamonds have inspired many things, and also
some controversy. One music CD on my bookshelf is "
Diamond Music" by
Karl Jenkins. Diamond is starting to become a useful
electronic material. Polished substrates are important to fabrication of electronic devices, so anything that advances that art is immediately useful.
References:
- Steven Miller, "Scratching Diamonds," Argonne National Laboratory, April 29, 2004.
- Earle Radcliffe Caley and John F.C. Richards, "Theophrastus on Stones: Introduction, Greek Text, English Translation, and Commentary," Ohio State University (Columbus, Ohio, 1956).
- John Bostock and H.T. Riley, "The Natural History. Pliny the Elder," Taylor and Francis (London, 1855), Book 37, chapter 76. Original Latin, here; translation, here.
Obsianae fragmenta veras gemmas non scariphant, in ficticiis scariphatio omnis candicat. iam tanta differentia est, ut aliae ferro scalpi non possint, aliae non nisi retuso, omnes autem adamante. plurimum vero in iis terbrarum proficit fervor.
Dust of Obsian stone will not leave a mark upon the surface of a genuine stone: but where the gem is artificial, every mark that is made will leave a white scratch upon it. In addition to this, there is such a vast diversity in their degrees of hardness, that some stones do not admit of being engraved with iron, and others can only be cut with a graver blunted at the edge. In all cases, however, precious stones may be cut and polished by the aid of adamas; an operation which may be considerably expedited by heating the graver.
- How to soften a diamond, Fraunhofer Institute for Mechanics of Materials Press Release, November 28, 2010.
- Lars Pastewka, Stefan Moser, Peter Gumbsch and Michael Moseler, "Anisotropic mechanical amorphization drives wear in diamond," Nature Materials, nmat2902, Published Online 28 November 28, 2010.
Permanent Link to this article
Linked Keywords: Diamond; Mohs scale of mineral hardness; cubic zirconia; quartz; Herkimer diamond; borazon; boron nitride; beta carbon nitride; Theophrastus; Pliny the Elder; Naturalis Historia; On Stones; chemical bonds; crystals; anisotropy; lattice; tribology; Fraunhofer Institute for Mechanics of Materials; Freiburg, Germany; Nature Materials; molecular dynamics modeling; quantum mechanics; carbon; orbital hybridisation; amorphous solid; oxidation; oxygen; carbon dioxide; blood_diamond; Diamond Music; Karl Jenkins; semiconductor; electronic material.