Amorphous Ice
March 27, 2023
I spent most of my
career at a major
aerospace company, and several members of our
materials science team had a background in
amorphous metals.
Thermodynamics will produce an
equilibrium mix of
phases in an
alloy, but only if that alloy is
annealed for long periods to allow the
atoms to
diffuse to conform to the equilibrium state. If you
quench your alloy without an anneal, a non-equilibrium state will be locked-in. This idea was taken to an extreme in the creation ofamorphous metals with desirable
physical properties by a technique known as
melt spinning.
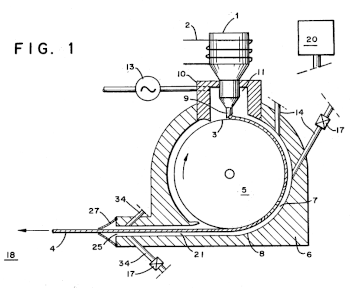
Fig. 1 of US Patent no. 4,592,411, "Method of and apparatus for continuously casting metal filament in a vacuum," by John R. Bedell and Howard H. Liebermann, June 3, 1986.
Molten metal in a crucible is induction heated and gravity fed through a nozzle onto a rapidly rotating copper flywheel.
The high thermal conductivity of copper keeps the surface of the flywheel close to room temperature. As a result, the liquid metal is very quickly solidified into an amorphous metal ribbon.
(Via Google Patents.[1] Click for larger image.)
Another method for creation of amorphous metals is
mechanical alloying.[2] This is accomplished by using a high
speed ball mill to repeatedly
pulverize and
cold weld elemental or pre-alloyed
powders. Such mechanical alloying was initially developed, not for the creation of amorphous metals, but for creation of advanced
nickel-based and
iron-based
superalloys for
turbine engine blades. The powders can then be made into
billets or
near-net-shape parts by
hot isostatic pressing.
This same ball-milling approach was used by a
research team from the
University College London (London, UK) and the
University of Cambridge (Cambridge, UK) to create an amorphous phase of
ice.[3-6] While ball-milling is regularly used to make amorphous materials, it's never been applied to ice.[4] This new phase of water could assist in explaining the strange
properties of liquid water.[5]
As I wrote in an
earlier article (Ice XVIII, June 24, 2019), there are many phases of water. The
solid forms of water include
Ice Ih, the ubiquitous
hexagonal crystalline form of
ice that we see on our automobile windshields;
Ice Ic, a form of ice in which the
oxygen atoms are arranged in the
diamond cubic structure;
ice II, a
rhombohedral crystalline form of ice;
ice III, a
tetragonal crystalline ice that's
denser than water;
ice IV, a
metastable rhombohedral phase of ice; and,
ice V, a
monoclinic crystalline phase of ice.
There are also
ice VI, a tetragonal crystalline phase of ice;
ice VII, a cubic phase of ice;
ice VIII a version of ice VII with a more orderly atomic arrangement,
ice IX, a tetragonal phase of ice;
ice X, a phase of ice that forms at about 70
GPa;
ice XI, an
orthorhombic crystalline form of ice that's the most stable configuration of ice I
h;
ice XII, a tetragonal, metastable, dense crystalline phase of ice that's about 1.3 times denser than water;
ice XIII, a
monoclinic crystalline phase of ice;
ice XIV, an orthorhombic crystalline phase of ice;
Ice XV, a phase of ice VI in which the
hydrogen nuclei are well ordered; and,
ice XVI, the ice having the least density.
Says
senior author of the amorphous ice paper,
Christoph Salzmann, a
professor of
chemistry at University College London, "Water is the foundation of all
life. Our existence depends on it, we launch
space missions searching for it, yet from a
scientific point of view it is poorly understood."[5] Water has many strange properties, the most significant of which is that it's most
dense at 4°C and it's less dense when it's
frozen.[5-6] As a consequence, ice
floats on water, and this property must have had a significant affect on life on
Earth.
compression of water becomes easier the more it is compressed, a
phenomenon contrary to that for most other
substances.[5]
Water can also become greatly
supercooled; that is, it will exist as a liquid many tens of
degrees Celsius below its freezing point.[6] In this supercooled state, liquid water might exist in two different phases, a high-density liquid and a low-density liquid.[5-6] Low density amorphous ice was discovered in the
1930s in an
experiment in which
water vapor was
condensed onto a surface at -110 degrees Celsius.[5] High density amorphous ice was discovered in the
1980s in experiments in which ordinary ice was compressed at a temperature of about -200 degrees Celsius.[5]
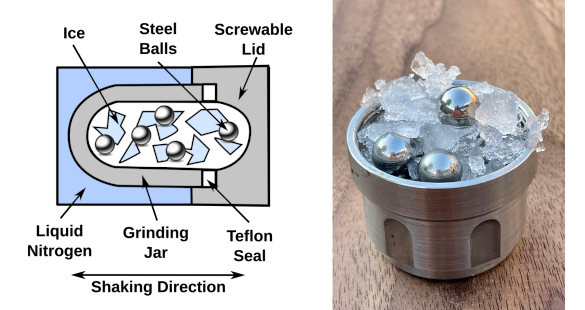
Shaken, not stirred. This is the apparatus used to make amorphous ice. (Left image, created from data in a University College London image using Inkscape and a Wikimedia Commons image of a steel ball. Right image, a University College London image by Christoph Salzmann.Click for larger image.)
Liquid water has a density between that of low-density and high-density amorphous ice.[3] The research team showed that ball milling ice-Ih, ordinary ice, at low temperature produces a medium-density amorphous ice (MDA) within this density
gap.[3-4] This amorphous ice looks like a fine white powder.[5] MDA might be the true
glassy state of liquid water.[3] The ball-milling process involved
shaking ice and
steel balls in a -200 degrees Celsius
container.[4-5] As lead author
Alexander Rosu-Finsen, who did the experiments while at University College London, states,
"We shook the ice like crazy for a long time and destroyed the crystal structure. Rather than ending up with smaller pieces of ice, we realized that we had come up with an entirely new kind of thing, with some remarkable properties."[5]
Verification of this new amorphous ice was done by multiple
analytical techniques. These were a density measurement using
buoyancy in
liquid nitrogen,
X-ray diffraction, and
Raman spectroscopy.[5] One remarkable property of medium-density amorphous ice is that there's a sharp increase of its
recrystallization enthalpy when it's compressed at low temperatures.[3]
Calorimetry showed that the medium-density amorphous ice recrystallized at warmer temperatures releases an extraordinary amount of heat.[4]
The research team was able to create a
computer simulation of the ball-milling process that created the amorphous ice. They
mimicked ball-milling using repeated
random shearing of crystalline ice.[4] Said co-author
Michael Davies, who did the computer simulation, "We found remarkable similarities between MDA and liquid water."[4]
Amorphous ice is common in
space, but it would occur only in the cold upper reaches of
Earth's atmosphere.[4-5] In the cold of space, there's not enough
thermal energy for the
molecules of water to rearrange themselves into a crystal.[5] Medium-density amorphous ice may exist inside ice
moons of
Jupiter and
Saturn, since
tidal forces will exert similar shear stress on ordinary ice as that created by ball milling.[4-5] The large recrystallization enthalpy of the amorphous ice could trigger
icequakes in the thick ice surface of moons such as
Ganymede.[5]
Funding for this research was provided by the
European Research Council and the
Engineering and Physical Sciences Research Council.[3]
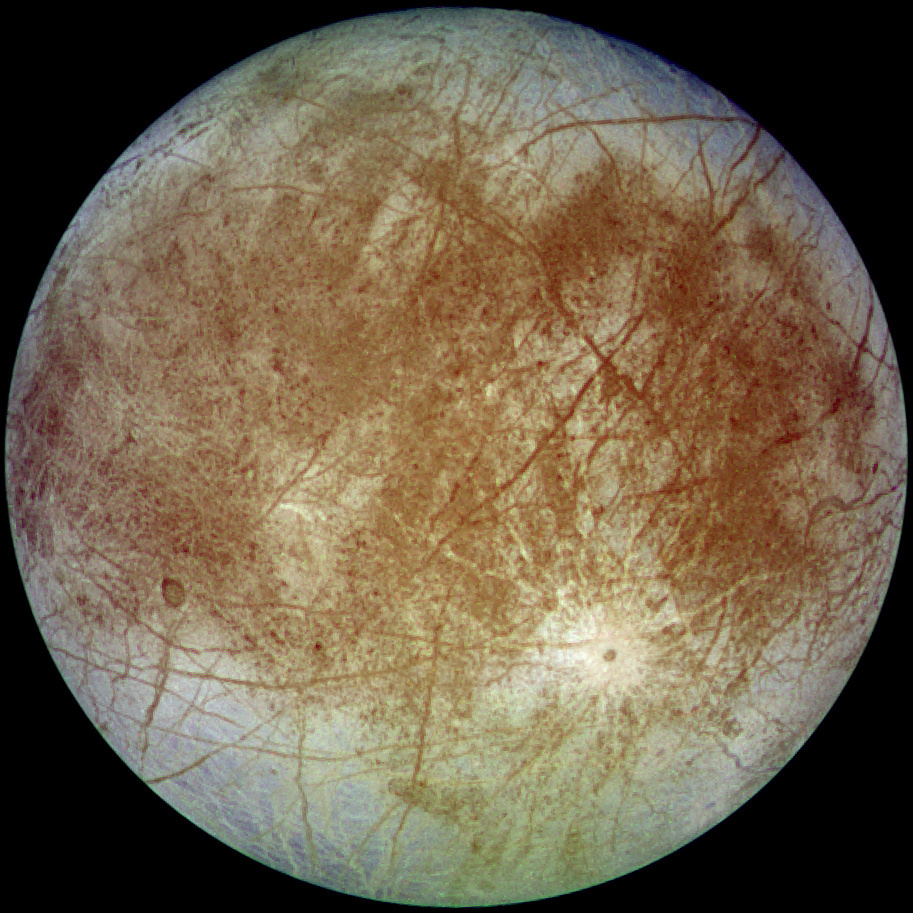
Europa, a moon of Jupiter.
Europa is the sixth-closest to the planet of the known moons of Jupiter, and the sixth-largest moon in the Solar System.
Europa's icy crust has a high light reflectivity, with an albedo of 0.64. Its surface appears to be active, which is indicative of the tidal forces caused by Jupiter.
(Wikimedia Commons image by NASA/JPL/DLR. Click for larger image.)
References:
- John R. Bedell and Howard H. Liebermann, "Method of and apparatus for continuously casting metal filament in a vacuum," US patent no. 4,592,411 (June 3, 1986).
- Mechanical Alloying, University of Cambridge.
- Alexander Rosu-Finsen, Michael B. Davies, Alfred Amon, Han Wu, Andrea Sella, Angelos Michaelides, Yusuf Hamied, and Christoph G. Salzmann, "Medium-density amorphous ice," Science, vol. 379, no. 6631 (February 2, 2023), pp. 474-478, DOI: 10.1126/science.abq2105.
- New form of ice is like a snapshot of liquid water, University of Cambridge Press Release, February 2, 2023.
- Keith Cowing, "Discovery Of Amorphous Ice May Change Our Understanding Of Water," University College London Press Release, February 2, 2023.
- Emily Conover, "Water is weird. A new type of ice could help us understand why," Science News, February 2, 2023.
Linked Keywords: Career; aerospace; company; materials science; amorphous metal; thermodynamics; thermodynamic equilibrium; phase (matter); alloy; annealing (metallurgy); annealed; atom; diffusion; diffuse; quenching; quench; physical property; melt spinning; US patent no. 4,592,411; patent; melting; molten; metal; crucible; induction heating; induction heated; gravity feed; gravity fed; nozzle; rotation; rotating; copper; flywheel; thermal conductivity; surface; room temperature; liquid; freezing; solidify; ribbon; Google Patents; mechanical alloying; speed; ball mill; pulverize; cold welding; cold weld; chemical element; elemental; powder (substance); nickel; iron; superalloy; turbine engine blade; billet; near-net-shape; hot isostatic pressing; research; University College London (London, UK); University of Cambridge (Cambridge, UK); ice; properties of liquid water; solid; Ice Ih; hexagonal crystal system; Ice Ic; oxygen; diamond cubic structure; ice II; trigonal crystal system; rhombohedral crystal; ice III; tetragonal crystal; density; dense; ice IV; metastable; ice V; monoclinic crystal; ice VI; ice VII; ice VIII; ice IX; ice X; pascal (unit); GPa; ice XI; orthorhombic crystal; ice XII; ice XIII; monoclinic crystal; ice XIV; Ice XV; hydrogen; atomic nucleus; ice XVI; author; Christoph Salzmann; professor; chemistry at University College London; life; spacecraft; space mission; science; scientific; buoyancy; float; Earth; compression (physics); phenomenon; chemical substance; supercooling; supercooled; degrees Celsius; 1930s; experiment; water vapor; condensation; condense; 1980s; amorphous ice apparatus; Shaken, not stirred; Inkscape; gap; glassy; vibration; shake; stee; ball; container; Alexander Rosu-Finsen; verification; analytical chemistry; liquid nitrogen; X-ray crystallography; X-ray diffraction; Raman spectroscopy; recrystallization; enthalpy; calorimetry; computer simulation; mimicked; randomness; random; shear stress; shearing; Michael Davies; outer space; Earth's atmosphere; thermal energy; molecule; natural satellite; moon; Jupiter; Saturn; tidal force; Ganymede (moon); funding of science; European Research Council; Engineering and Physical Sciences Research Council; Europa (moon); planet; Solar System; crust (geology); light reflectivity; albedo; lithosphere; Wikimedia Commons.