Rare Earths from Bacteria
May 29, 2023
Many
plants concentrate trace elements. One example is
locoweed, the common name for plants of the
species Astragalus, which concentrate
selenium up to several
percent of their
dry weight.[1]
Nickel is a valued
metal (Nickel is about a hundred times more
expensive than
iron), and
Pycnandra acuminata, a species of plant in the
family,
Sapotaceae, concentrates nickel from the nickel-rich
soil of
New Caledonia. The dry weight of its
sap contains up to 25% nickel
citrate, and the sap has a
turquoise-green color as a consequence.
_and_Pycnandra acuminata.jpg)
Left, Astragalus lentiginosus, also known as the "spotted locoweed." Right, Pycnandra acuminata
Brazil nuts, the seeds of Bertholletia excelsa in the family Lecythidaceae, concentrate selenium up to 83 micrograms per gram (shell included).[2] Toxic effects of selenium start at about 700 micrograms; so, don't eat too many Brazil nuts at one time.
(Left, Wikimedia Commons image by Stan Shebs. Right, a Wikimedia Commons image by Benoit Henry. Click for larger image.)
Bacteria are known to concentrate
toxic heavy metals, and that's inspired
genetic engineers in their attempts to maximize this
trait. While the main purpose is for
bioremediation, such
bioaccumulation can be a means of
extracting valuable heavy metals for reuse, rather than
disposal.[3] In particular, the
sulfate-
reducing bacteria,
Desulfovibrio desulfuricans, will reduce
U6+ to U
4+ to provide an easy means of recovering
uranium from
contaminated waters and
waste streams.[4] Also interesting are
rare earth concentrating bacteria, as discussed later in this article.
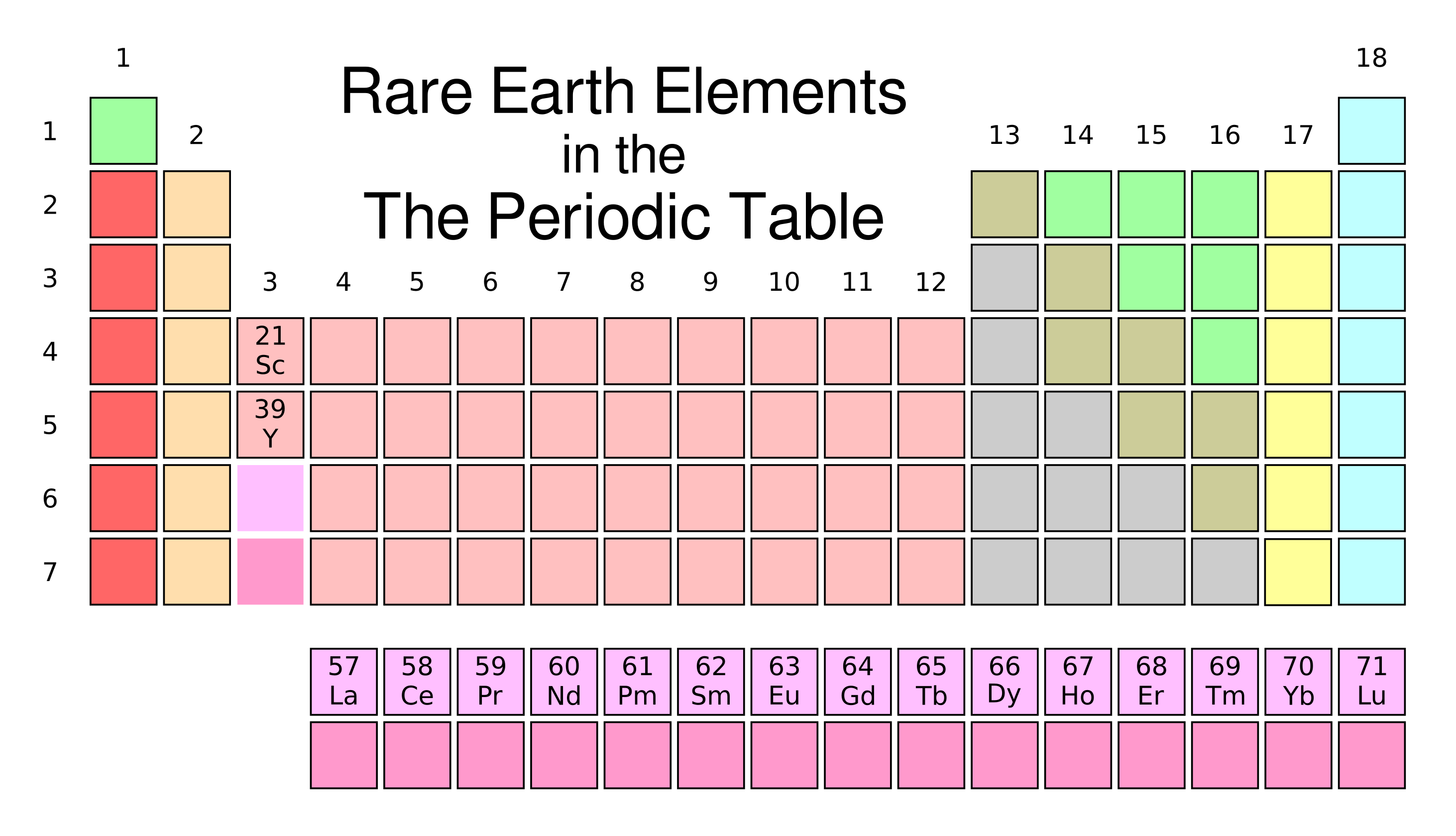
A good test of an inorganic chemist is whether he or she can name all of these uncommon elements while looking at just their symbols on the periodic table. The most technologically useful rare earths are yttrium (Y), lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), ytterbium, and lutetium (Lu). (Created using Inkscape. Click for larger image.)
Rare earth
elements are used in many
industrial and
consumer products. These include
catalysts, the major use, the
magnets used in
electric motors for
drones and
automobile windows,
laser crystals, and
phosphors. The estimated value of
United States imports of rare-earth
compounds and
metals was $160 million in 2021, a significant increase from $109 million in 2020.[5]
China's 2021 production in
tons of rare earth
oxide equivalents was 168,000, followed by the United States at 43,000,
Myanmar (Burma) at 26,000, and
Australia at 22,000.[5]
China controls the world market in rare earth
metals, and it has been limiting
exports of these elements. As a consequence, other countries have been searching for new sources of these metals. Just this year, reserves of one million
metric tons were discovered in
Kiruna, Sweden. Rare earth
deposits have been discovered in
seafloor mud in the
Pacific Ocean.[6-7] As I wrote in a
previous article (Rare Earths from Coal Waste, April 18, 2022),
coal ash, a
byproduct of
coal combustion, contains rare earth elements.[8-9]
Acid mine drainage contains
sulfuric acid that
dissolves rare earths from surrounding
rocks. An estimated 6,000 metric tons of rare earths are created
annually in acid mine drainage sites.[10-11]
A team of
German scientists from the
Technical University of Munich (Garching, Germany), and the
University of Applied Sciences Kaiserslautern (Pirmasens, Germany) have found that some
exotic photosynthetic cyanobacteria efficiently absorb rare earths from
wastewater, and the rare earths can be
extracted from their
biomass.[12-14] Their findings have been
published in an
open access paper in the
journal,
Frontiers in Bioengineering and Biotechnology.[12]
One fundamental problem in rare earth
extraction from
ore is that they are
chemically similar. As a consequence, their extraction requires
environmentally toxic chemicals and a lot of
energy.[12] Rare earth recovery from
industrial wastewater, and acid mine drainage, as mentioned above, is becoming an encouraging alternative.[12] Biosorption of metal ions by
photosynthetic microorganisms, a
metabolically passive and rapid process that involves the
reversible binding of
ions from
aqueous solutions to biomass is a viable alternative method for both bioremediation and metal recovery.[12,14] The present study builds from 2021 research of heavy metal removal by cyanobacteria.[13]
Says study's
first author,
Michael Paper, a scientist at the Technical University of Munich,
"We found that biomass derived from cyanobacteria has excellent adsorption characteristics due to their high concentration of negatively charged sugar moieties, which carry carbonyl and carboxyl groups. These negatively charged components attract positively charged metal ions such as REEs, and support their attachment to the biomass."[14]
Seven
terrestrial and five
aquatic cyanobacterial strains were investigated for their absorption of rare earths from aqueous solution.[13-14] They are found in
habitats with harsh environmental conditions, such as the
arid soils in
Namibian deserts, rock
crevices in
South Africa,
natron lakes in
Chad, and
polluted streams in
Switzerland.[13-14] Most of these had never been checked for their bioremediation potential.[13-14]

If it's green, it must be biology - Professor Thomas Brück taking samples at the cyanobacteria photobioreactor for extraction of rare earth elements.
There's a laboratory adage as follows: If it's green, it's biology; if it stinks, it's chemistry; and, if it doesn't work, it's physics. (TUM image by Andreas Heddergott, released under the Creative Commons Attribution License. Click for larger image.)
The rare earth elements,
cerium, neodymium,
terbium, and lanthanum, were used to screen the adsorption capacity of the bacteria, and a not previously
investigated new species of
Nostoc, Nostoc sp. 20.02, showed the highest adsorption capacity with 84.2-91.5 mg/g.[12] This is about 10% of the bacterial
dry mass. Others showed adsorption capacities of 47.2 to 83.5 mg/g.[12] Absorption was rapid at the mildly acidic
pH of 5-6, but decreased at more acidic pH.[12,14] Most of the cerium in solution was captured within five minutes.[13] Says study author,
Thomas Brück,
"The cyanobacteria described here can adsorb amounts of REEs corresponding to up to 10% of their dry matter. Biosorption thus presents an economically and ecologically optimized process for the circular recovery and reuse of rare earth metals from diluted industrial wastewater from the mining, electronic, and chemical-catalyst producing sectors."[14]
It was observed that rare earth biosorption was possible even at low metal concentration, and the process was more efficient when there were no competing
cations, such as
zinc,
lead,
nickel, or
aluminum.[13-14] Overall, the application of cyanobacteria in bioremediation and recovery of rare earth elements is a promising method for the development of an industrial process, but needs to be to be further optimized and adjusted.[12] In a subsequent project, the research team plans to conduct more experiments on a larger scale with a view to industrial application of the results.[13]
References:
- John A. Izbicki and T.F. Harms, "Selenium Concentrations in Leaf Material from Astragalus Oxyphysus
(Diablo Locoweed) and Atriplex Lentiformis (Quail Bush) in the Interior Coast Ranges and the Western San Joaquin Valley, California," U.S. Geological Survey Water-Resources Investigations Report 86-4066, 1986 (PDF File).
- Christine D Thomson, Alexandra Chisholm, Sarah K McLachlan, and Jennifer M Campbell, "Brazil nuts: an effective way to improve selenium status," The American Journal of Clinical Nutrition, vol. 87, no. 2 (February 1, 2008), pp. 379-384, https://doi.org/10.1093/ajcn/87.2.379.
- Patrick Diep, Radhakrishnan Mahadevan, and Alexander F. Yakunin, "Heavy Metal Removal by Bioaccumulation Using Genetically Engineered Microorganisms," Frontiers in Bioengineering and Biotechnology, vol. 6 (October 1, 2018), Article no. 157, doi: 10.3389/fbioe.2018.00157 (PDF File).
- Derek R. Lovley and Elizabeth J. P. Phillips, "Reduction of Uranium by Desulfovibrio desulfuricans," Applied and Environmental Microbiology, vol. 58, no. 3 (March, 1992), pp. 850-856 (PDF File).
- Rare Earths, U.S. Geological Survey, Mineral Commodity Summaries, January 2022 (PDF File).
- Yasuhiro Kato, Koichiro Fujinaga, Kentaro Nakamura, Yutaro Takaya, Kenichi Kitamura, Junichiro Ohta, Ryuichi Toda, Takuya Nakashima, and Hikaru Iwamori, "Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements," Nature Geoscience, vol. 4, no. 8 (August, 2011), pp. 535-539, doi:10.1038/ngeo1185.
- Nicola Jones, "Sea holds treasure trove of rare-earth elements," Nature News. July 3, 2011, doi:10.1038/news.2011.393.
- Report on Rare Earth Elements from Coal and Coal Byproducts. Report to Congress, United States Department of Energy, January, 2017 (PDF File).
- Clint Scott and Allan Kolker, "Rare earth elements in coal and coal fly ash: U.S. Geological Survey Fact Sheet 2019-3048," U.S. Geological Survey (Reston, Virginia), September 12, 2019, 4 pages, https://doi.org/10.3133/fs20193048.
- Jennifer Wilcox, "Creating a mineral supply chain from mining wastes,Pittsburgh Post-Gazette, February 26, 2022.
- Maddie Stone, "The plan to turn coal country into a rare earth powerhouse," grist.com, May 26, 2021.
- Michael Paper, Max Koch, Patrick Jung, Michael Lakatos, Tom Nilges, and Thomas B. Brück, "Rare earths stick to rare cyanobacteria: Future potential for bioremediation and recovery of rare earth elements," Frontiers in Bioengineering and Biotechnology, vol. 11 (February 28, 2023), Article no. 1130939, DOI: 10.3389/fbioe.2023.1130939. This is an open access paper with a link to a PDF file at the same URI.
- Previously uninvestigated strains of cyanobacteria can adsorb metals, Technical University of Munich Press Release, February 28, 2023.
- 12 exotic bacteria found to passively collect rare earth elements from wastewater, Frontiers in Bioengineering and Biotechnology Press Release, February 28, 2023.
- Abdullah Al-Amin, Fahmida Parvin, Joydeep Chakraborty, and Yong-Ick Kim," Cyanobacteria
mediated heavy metal removal: A review on mechanism, biosynthesis, and removal
capability," Environ. Technol. Rev., vol. 10, no. 1 (January 12, 2021), pp. 4457, doi:10.1080/21622515.2020.186932.
Linked Keywords: Plant; concentration; concentrate; trace element; locoweed; species; Astragalus; selenium; percentage; percent; dry matter; dry weight; nickel; metal; cost; expensive; iron; Pycnandra acuminata; family (biology); Sapotaceae; soil; New Caledonia; sap; citric acid; citrate; turquoise-green color; Brazil nut; seed; Lecythidaceae; Bertholletia excelsa; gram; microgram; nutshell; shell; toxicity; toxic effect; Wikimedia Commons; Stan Shebs; bacteria; toxic heavy metal; genetic engineering; genetic engineer; phenotypic trait; bioremediation; bioaccumulation; extraction (chemistry); extracting; waste management; disposal; sulfate; redox; reducing; Desulfovibrio desulfuricans; uranium; water pollution; contaminated water; waste stream; rare earth element; Rare Earth Elements in Periodic Table; inorganic chemistry; inorganic chemist; chemical element; element; symbol; periodic table; technology; technologically; yttrium; lanthanum; cerium; praseodymium; neodymium; samarium; europium; gadolinium; terbium; dysprosium; ytterbium; lutetium; Inkscape; chemical element; industry; industrial; consumer; product (business); catalysis; catalyst; rare earth magnet; electric motor; unmanned aerial vehicle; drone; automobile; window; laser; crystal; phosphor; United States; import; chemical compound; metal; People's Republic of China; China; ton; oxide; Myanmar (Burma); Australia; market (economics); export; metric ton; Kiruna, Sweden; mineral deposit; seafloor; mud; Pacific Ocean; fly ash; coal ash; byproduct; coal; combustion; acid mine drainage; sulfuric acid; dissolution (chemistry); dissolve; rock (geology); year; annually; Germany; German; scientists; Technical University of Munich (Garching, Germany); University of Applied Sciences Kaiserslautern (Pirmasens, Germany); exotic; photosynthesis; photosynthetic; cyanobacteria; efficiency; efficiently; absorption (chemistry); absorb; wastewater; extraction (chemistry); extract; biomass; scientific literature; publish; open access journal; open access paper; scientific journal; Frontiers Media; Frontiers in Bioengineering and Biotechnology; extractive metallurgy; extraction; ore; chemistry; chemically; environment (biophysical); environmentally; toxicity; toxic chemical; energy; industry; industrial; photosynthesis; photosynthetic; microorganism; metabolism; metabolically; passivity (engineering); passive; reversible process (thermodynamics); reversible; chemical bond; binding; ion; aqueous solution; first author; Michael Paper; electric charge; negatively charged; sugar; moiety; moieties; carbonyl; functional group; positively charged; soil; terrestrial; aquatic ecosystem; habitat; arid; Namibia; Namibian; desert; fracture (geology); crevice; South Africa; natron; lake; Chad; water pollution; polluted; stream; Switzerland; cyanobacteria photobioreactor; biology; Professor; Thomas Brück; sample (material); bioreactor; extractive metallurgy; extraction; laboratory; adage; odor; stink; chemistry; physics; Andreas Heddergott; Creative Commons Attribution License; research; investigated; Nostoc; pH; Thomas Brück; economics; economically; ecology; ecologically; engineering optimization; optimized process; mining; electronic; cation; zinc; lead; nickel; aluminum.