Atmospheric Water Harvesting
July 11, 2022
Tikalon's
home in
Northern New Jersey is blessed with abundant
rainfall, which leads to lush
vegetation (and
pollen allergies!). Indeed,
New Jersey is known as the
Garden State. However, other parts of the
United States and much of the
world are facing
water scarcity. I wrote about water scarcity in an
earlier article (Future Water Scarcity, March 28, 2016).
The
United Nations has compiled a list of water
statistics, as follows.[1]
• 72% of all water withdrawals are used by agriculture, 16% by municipalities for services and households, and 12% by industry.
• Five out of 11 regions withdraw 25% more water than is replenished by renewable freshwater resources, such as rainfall.
• 2.3 billion people live in water-stressed countries, and 733 million of these live in highly water-stressed countries.
• 1.2 billion people, about a sixth of the world's population , live in severely water-constrained agricultural areas.
• Today, 1.42 billion people, including 450 million children, live in areas of high or extremely high water vulnerability.
• About 4 billion people, nearly two-thirds of the world's population, experience severe water scarcity during at least one month of the year. This could increase to up to 5.7 billion in 2050.
• 700 million people worldwide could be displaced by intense water scarcity by 2030.
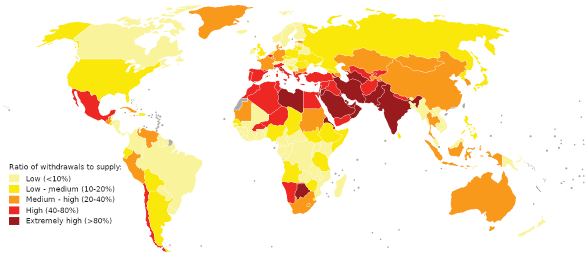
World water stress map for 2019. Sources: World Resources Institute, National Water Stress Rankings, and World Resources Institute, Projected Water Stress Country Rankings. (Wikimedia Commons image by Genetics4good. Click for larger image.)
Adult humans are more than 50% water by
weight, while nearly 75% of an
infant is water. A human living in a
desert climate needs to consume several
gallons of water each day to survive. Increased
industrial activity has led to significant
pollution of water sources in
developing countries.
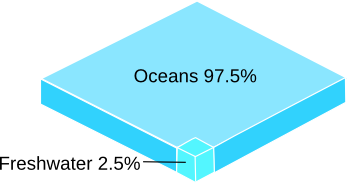
The Earth has a lot of water, but most of it is seawater.
Desalination of saline water, typically by reverse osmosis, helps to mitigate water shortage along sea coasts.
In reverse osmosis, pressure on one side of a semipermeable membrane induces water permeation through the membrane while rejecting salts.
(Rendered using Inkscape.)
Most
mornings, I'm reminded that there's a lot of water in the
air. Cool
summer mornings find my
lawn covered in a thick
blanket of
dew. My
automobile is likewise covered with a thick layer of water,
condensed from the
atmosphere. The same is true on
winter mornings when the dew appears as a thick layer of
ice. In an
earlier article (Fog Water Harvesting, December 2, 2010), I did a
back-of-the-envelope calculation of how effective my automobile is at collecting dew.
I estimate that after a heavy dew, the
surface of my car is covered by about a
liter of water.
Approximating the
geometry of the car as two
rectangular parallelepipeds, one atop the other, having about 220 square feet exposed to the air (about 20
square meters) when the
undercarriage is ignored. This results in a collection
efficiency of about 50
milliliters per square meter.
Trees, which have
leaves or
needles with a large collecting area hoisted high into the air, are natural
fog water harvesters. This illustrates the
idea that
potable water can be harvested from the air.
Retreating from the idea that you need a cool night to harvest water from the air, you can use
electrical refrigeration to
condense water vapor through
cooling. This is an
energy-intensive
process, but it could be enabled by storing
solar energy during the day.
Plastic netting is a more effective fog water harvester than automobile
metal and
glass, since air can
flow though it. Fog water harvesting has been demonstrated using large plastic nets in
Lima, Peru.[2] During the cooler months of
May through
November, 32
square meter nets have captured as much as 590
liters in a single day.[2] A double-net system produced an
average of 300 liters per day throughout the year, with 2650 liters being produced in a single day.[2]
Scientists have been
researching better atmospheric water harvesting
materials. Recently, a team of scientists and
mechanical engineers from the
University of Texas at Austin (Austin, Texas) have developed an
inexpensive gel film, created from abundant materials, that's capable of extracting water from air in even the
driest climates.[3-4] Their results are published as an
open access article in
Nature Communications.[3]
As
chemists know, some salts, such as
lithium chloride (LiCl),
calcium chloride (CaCl2), and
magnesium chloride (MgCl2) are
hygroscopic, and they have high water uptake at even low
relative humidity. My
parents had a primitive
dehumidifier in the
cellar of their house that used calcium chloride as the hygroscopic agent. However, the
aggregation of salt
crystals during
hydration creates a
passivation layer, so the
water cycling performance is reduced.[3]
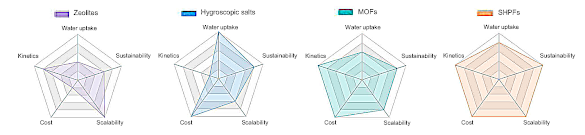
Radar chart comparison of atmospheric water harvesting technologies. (Fig. 1d of ref. 3, licensed under the Creative Commons Attribution 4.0 International License.[3] Click for larger image.)
The material used in the present study is made from
cellulose,
konjac glucomannan, a water-
soluble polysaccharide that's a
food additive used as an
emulsifier and
thickener, and LiCl.[3-4] The
open-pore structure of the glucomannan increases the rate of the moisture-capturing process, while the cellulose becomes
hydrophobic when heated to assist in the water removal.[4] The material is a super hygroscopic polymer film with an
hierarchical porous structure with sorbent-air
interfaces and rapid water vapor
transport pathways.[3]
The research builds on previous work by the same research team on a super moisture-absorbent gel composed of hygroscopic
polypyrrole chloride penetrating an hydrophilic-switchable polymer network of
poly N-isopropylacrylamide.[5-6] This
super sponge can collect large amounts of water from the atmosphere, and removing the water merely involves heating by exposure to sunlight for a few minutes.[6] The reaction to produce the cellulose-glucomannan material is so easy it can be done at home.[4] The resultant gel film,
freeze-dried after
casting in a
mold, is
flexible, it can be created in a desired
shape, and it's created in just two minutes.[4]
The starting materials for creation of the cellulose-glucomannan material cost just $2 per
kilogram. A single kilogram can produce more than 6 liters of water per day in areas with less than 15% relative humidity and 13 liters per day at 30% relative humidity.[4] Six liters seems like a small amount, but thicker films and arrays of films will enhance the
yield.[4] This material can be used at 14-24 cycles per day in arid environments to produce a water yield of 5.8-13.3 L/kg.[3] The 14 daily cycles are realized at 15% relative humidity, and the 24 daily cycles at 30% relative humidity.[3] The 5.8 L/kg yield is obtained at 15% relative humidity, and 13.3 L/kg yield is obtained at 30% relative humidity.[3] This research was funded by the
Defense Advanced Research Projects Agency (DARPA).[4]
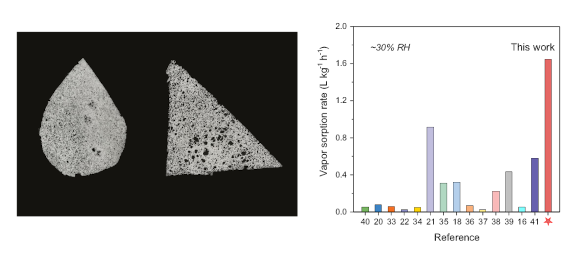
Left, the atmospheric water harvesting material can be molded into different shapes. Right, a comparison of atmospheric water harvesting materials at ∼30% relative humidity. (Left, a University of Texas image. Right, figure 3f of ref. 3, licensed under the Creative Commons Attribution 4.0 International License. Click for larger image.)
References:
- United Nations, Water Scarcity
- Gaia Vince, "News Focus/Hydrology-Out of the Mist," Science, vol. 330, no. 6005 (November 5, 2010), pp. 750-751, DOI: 10.1126/science.330.6005.750.
- Youhong Guo, Weixin Guan, Chuxin Lei, Hengyi Lu, Wen Shi, and Guihua Yu, "Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments," Nature Communications, vol, 13 (May 19, 2022), Article no. 2761, https://doi.org/10.1038/s41467-022-30505-2. This is an open access paper with a PDF file here.
- Low-Cost Gel Film Can Pluck Drinking Water From Desert Air, University of Texas at Austin Press Release, May 23, 2022.
- Fei Zhao, Xingyi Zhou, Yi Liu, Ye Shi, Yafei Dai, and Guihua Yu, "Super Moisture-Absorbent Gels for All-Weather Atmospheric Water Harvesting," Advanced Materials, vol. 31, no. 10 (March 8, 2019), https://doi.org/10.1002/adma.201806446.
- Solar-Powered Moisture Harvester Collects and Cleans Water from Air, University of Texas at Austin Press Release, March 13, 2019.
Linked Keywords: Home; Morris County, New Jersey; Northern New Jersey; rain; rainfall; vegetation; allergic rhinitis; pollen allergy; New Jersey; nickname; Garden State; United States; world; water scarcity; United Nations; statistics; agriculture; municipality; service (economics); household; industry; renewable resource; freshwater; human; people; country; world population; child; children; month; year; 2019 world water stress map; world; water scarcity; water stress; map; water stress ranking; Wikimedia Commons; Genetics4good; adult; weight; infant; desert climate; gallon; industry; industrial; water pollution; pollution of water sources; developing country; Earth; water; seawater; desalination; saline water; reverse osmosis; sea coast; pressure; semipermeable membrane; permeation; salt (chemistry); Inkscape; morning; atmosphere of Earth; air; summer; lawn; blanket; dew; automobile; condensation; condense; atmosphere; winter; ice; dew harvesting; back-of-the-envelope calculation; surface; liter; approximation; approximate; geometry; cuboid; rectangular parallelepiped; square foot; square meter; chassis; undercarriage; efficiency; milliliter; tree; leaf; leaves; pine; foliage; needle; fog collection; fog water harvester; idea; drinking water; potable water; electricity; electrical; refrigeration; condensation; condense; water vapor; cooling; energy; thermodynamic process; solar energy; plastic; net (device); netting; metal; glass; airflow; Lima, Peru; month; May; November; liter; average; scientist; research; researching; material; mechanical engineering; mechanical engineer; University of Texas at Austin (Austin, Texas); cost; inexpensive; gel; film; arid; driest climate; open-access journal; open access article; Nature Communications; chemist; lithium chloride (LiCl); calcium chloride (CaCl2); magnesium chloride (MgCl2); hygroscopy; hygroscopic; relative humidity; parent; dehumidifier; basement; cellar; particle aggregation; crystal; hydrate; hydration; passivation (chemistry); passivation layer; chemical kinetics; water cycling performance; Spider graph; radar chart; atmospheric water harvesting; technology; Creative Commons Attribution 4.0 International License; cellulose; konjac glucomannan; solubility; soluble; polysaccharide; food additive; surfactant; emulsifier; thickening agent; porosity; open-pore structure; hydrophobe; hydrophobic; hierarchy; hierarchical; interface (chemistry); diffusion; transport pathway; polypyrrole chloride; poly N-isopropylacrylamide; freeze-drying; freeze-dried; casting; molding (process); mold; stiffness; flexible; geometry; shape; kilogram; yield (chemistry); Defense Advanced Research Projects Agency (DARPA); ccomparison of atmospheric water harvesting materials.