Mistletoe Glue
August 15, 2022
One of the most important items in my
laboratory is
five-minute epoxy, and this is likely the case for most
experimental physicists. Any
experiment includes
disparate components that need to be combined, and this epoxy is often the best expedient. Physicists in times before the
invention of five-minute epoxy made frequent use of
sealing wax as an instant
glue. I've also used
UV-curable photopolymers when five minutes was still too long to wait.
Optical physicists have solved this problem in a more elegant fashion through the invention of the
optical table.
Sometimes,
adhesives are not required, as
interatomic forces can be used to hold together objects that are much larger than
atoms.
Gauge blocks are
ceramic or
metal blocks that have been
polished to extreme
flatness and
smoothness, and this polishing allows the blocks to be joined together with atomic forces in contact. These blocks are known by
machinists as
Jo Blocks in honor of their
originator,
Swedish inventor,
Carl Edvard Johansson (1864-1943).
While
working at a
rifle factory, Johansson modified his
wife's sewing machine to do
grinding and
lapping, and he
fabricated blocks in his own
home. After promising results, Johansson persuaded his
employer to
fund further
development, and he received a Swedish
patent entitled, "Gauge Block Sets for
Precision Measurement," in 1901. He started his own
company in 1917, moved the company to the
United States, and sold the company to
Ford in 1923 to form its Johansson division. He was
posthumously awarded a
gold medal by the
Royal Swedish Academy of Engineering Sciences in 1943.
A simple
model of atomic force was proposed in 1929 by
physicist,
John Lennard-Jones (1894-1954).[1-2] The
equation for the
Lennard-Jones potential models the
repulsive force that exists when
non-bonding electron orbitals overlap as the
inverse twelfth
power of
distance, and the
attractive force, the
van der Waals force, as the inverse sixth power of the distance. The resultant
curve (in blue) is shown in the following figure.
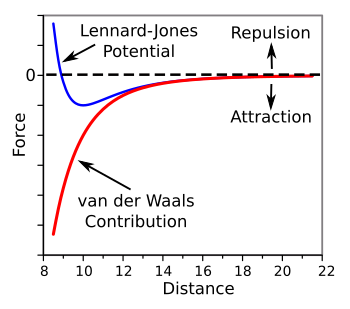
Lennard-Jones potential with its van der Waals force contribution.
The Lennard-Jones potential as a function of separation for an arbitrary molecule is shown in blue.
The force, which is attractive force below the dotted line, tapers to zero at large distances.
As is true in many cases, Aristotle (384-322 BC) had a similar observation. As he wrote in his Meteorology (11.1069a), "...where there is no contact, there is no coalescence."[3]
(Graph rendered using Gnumeric. Click for larger image.)
While the repulsive force in the Lennard-Jones potential is quite understandable as
like-charged electrons repelling each other, the attractive contribution is harder to understand. It arises from
electrostatic attraction between
induced dipoles at the
material surfaces. This attraction has a strength that's
proportional to the
polarizability of the material molecules.
This force was
elucidated by
Dutch physicist,
Johannes Diderik van der Waals (1837-1923), who was
awarded the 1910
Nobel Prize in Physics "for his work on the
equation of state for
gases and
liquids." The
van der Waals force is still important more than a
hundred years later, since a search for
papers on
arXiv with "van der Waals" in the
title gives 1,551 results at this writing.

Johannes Diderik van der Waals (1837-1923)
Van der Waals won the 1910 Nobel Prize in Physics "for his work on the equation of state for gases and liquids."
The idea of such a force was a part of his 1873 thesis in which he attributed the differences between real gases and an ideal gas to the existence of intermolecular interactions.
Minor planet 32893 van der Waals is named in his honor.
(Image modified for artistic effect, via Wikimedia Commons.)
How does this
abstract physics relate to anything in the real world? As a
child living in a
wooded suburban area of
Upstate New York, some of my
playtime patina was the result of
sticky pine sap and
milkweed sap. As it turns out, the van der Waals force is responsible for the stick in such sticky substances that we normally encounter.
In the
inaugural issue of
PNAS Nexus, an
Open access journal of the
National Academy of Sciences,
scientists from the
Max Planck Institute of Colloids and Interfaces (Potsdam, Germany) and
McGill University (Montreal, Quebec, Canada) have investigated the adhesive properties of
mistletoe viscin, a
natural cellulosic adhesive. This substance's
skin adhesion makes it a candidate as a
wound sealant.[4-5]

Mistletoe viscin is also known as birdlime, since this sticky substance is smeared on tree branches to trap birds.
Mistletoe was used by the Roman mythological hero, Aeneas, as a way to reach the underworld.
In the last few centuries, mistletoe is a Christmas decoration under which lovers are expected to kiss.
(A modified Wikimedia Commons image from a 1902 book, "A story from the Philippines," by Katherine Elizabeth Driscoll, also found at archive.org. The associated text reads, "And once Morris crept up and caught Tween Anne under the mistletoe just as papa, used to catch Mother-dear." Click for larger image.)
The
European mistletoe (Viscum album) is a
hemiparastic plant species, and it was used as a
medicine for various
ailments at the time of the
ancient Greeks, and possibly earlier.[4] Mistletoe
berries can produce a sticky viscin
thread up to two
meters in length.[5] This
fiber-reinforced adhesive has an
evolutionary advantage for
seed dispersal of this parasitic plant, since it allows the mistletoe seeds to stick to and
infest host plants.[4-5] Viscin is comprised of
hierarchically organized
cellulose microfibrils embedded in a
humidity-responsive
matrix.[4]
Deficiencies in many
synthetic adhesives include
irreversible adhesion and lack of adhesion under wet conditions.[4] That's why
Matthew Harrington, an
associate professor in the
Department of Chemistry at McGill University and a member of the mistletoe research team, was intrigued with his first encounter with mistletoe. Says Harrington, "I had never seen mistletoe before living in
Germany... So, when my
daughter was playing with a berry from a mistletoe we bought from a local
Christmas market, and it started sticking to everything, I was intrigued."[5]
A previous study demonstrated that the
stiffness of viscin fiber is highly tunable through changes in
relative humidity.[4] Fiber stiffness was as large as 20
GPa near 0% relative humidity, and this was reduced to about 300
MPa at relative humidity close to 95%.[4] Above 50% relative humidity, the fibers will
flow under
strain, but at low relative humidity the ultimate strain value was less than 2%.[4] Those properties allowed simple
processing in which wet viscin fibers could be stretched into
thin films or assembled into
3-D printing
three-dimensional structures (see photograph).[5]
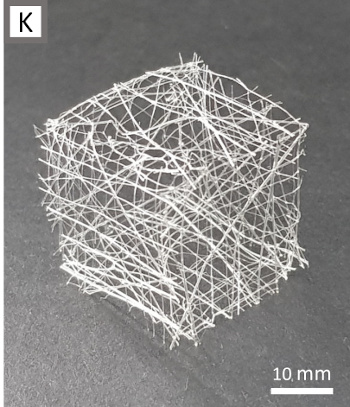
A cube formed from viscin thread.
Premanufactured two-dimensional meshes were fused by rehydration to form the three-dimensional object.
(Fig. 3k of ref. 4, distributed under a Creative Commons Attribution License.[4])
The
hygroresponsive behavior of the fibers is fully
reversible, and the
mechanical properties at a given relative humidity were
reproducible.[4] The viscin fibers have humidity-activated self-adhesive properties that allow
contact welding into complex
two-dimensional and three-dimensional shapes under
ambient conditions.[4] Stiff and
transparent free-standing films of viscin can be created by
biaxial stretching in the hydrated state, followed by
drying.[4] The viscin will adhere strongly to synthetic materials, such as
metals,
plastics, and
glass, and also
biological tissues, such as
skin and
cartilage.[4]
Skin adhesion makes viscin a candidate as a wound sealant, and its ability to stick to things reversibly under humid conditions makes it a candidate for many other applications.[4-5] Says
Nils Horbelt, a recently
graduated doctoral student at the Max Planck Institute and the
first author on the paper, "I wore a thin film of viscin on my skin for three days to observe its adhesive qualities and was able to remove it from my
fingers afterwards by simply
rubbing them together."[5] The abundance of mistletoe plants, and their
biodegradability and
renewability, are an added benefit.[5]
References:
- J. E. Lennard-Jones, "The Electronic Structure of Some Diatomic Molecules," Trans. Faraday Soc., vol. 25 (1929), pp. 668-686, DOI: 10.1039/TF9292500668.
- G.G. Hall, "The Lennard-Jones paper of 1929 and the foundations of molecular orbital theory,"Advances in Quantum Chemistry, vol. 22, (1991), pp. 1-6; also available, here.
- Aristotle, Hugh Tredennick, Trans., vols. 17-18, Harvard University Press (Cambridge, Massachusetts), 1933, via Tufts University Project Perseus.
- Nils Horbelt, Peter Fratzl, and Matthew J Harrington, "Mistletoe viscin: a hygro- and mechano-responsive cellulose-based adhesive for diverse material applications," PNAS Nexus, vol. 1, no. 1 (March 16, 2022), Article no. pgac026, pp. 1-11, https://doi.org/10.1093/pnasnexus/pgac026. This is an open access publication with a PDF file available at the article link.
- A biological super glue from mistletoe berries?, McGill University Press Release, June 14, 2022.
Linked Keywords: Laboratory; five-minute epoxy; experiment; experimental; physicist; disparate; component; invention; sealing wax; adhesive; glue; UV curing; UV-curable; photopolymer; optical physics; optical physicist; optical table; intermolecular force; interatomic force; atom; gauge block; ceramic; metal; cuboid; block; polishing; polished; flatness (manufacturing); smoothness; machinist; inventor (patent); originator; Sweden; Swedish; Carl Edvard Johansson (1864-1943); employment; working; rifle; factory; wife; sewing machine; grinding (abrasive cutting); lapping; manufacturing; fabricate; home; employer; funding of science; research and development; patent; precision; measurement; company; United States; Ford Motor Company; posthumous award; gold; medal; Royal Swedish Academy of Engineering Sciences; mathematical model; John Lennard-Jones (1894-1954); equation; Lennard-Jones potential; Coulomb's law; repulsive force; molecular orbital; non-bonding electron orbitals; multiplicative inverse; exponentiation; power; distance; attractive force; van der Waals force; curve; molecule; Aristotle (384-322 BC); Meteorology (Aristotle); coalescence (physics); Gnumeric; charge (physics); electron; electrostatic attraction; electric dipole moment; induced dipole; material; surface; proportionality (mathematics); polarizability; elucidate; Dutch; Johannes Diderik van der Waals (1837-1923); award; Nobel Prize in Physics; equation of state; gas; liquid; century; hundred years; scientific literature; paper; arXiv; title (publishing); thesis; ideal gas; intermolecular interaction; Minor planet; 32893 van der Waals; Wikimedia Commons; pure research; child; forest; wooded; suburban; Upstate New York; play (activity); playtime; patina; adhesion; sticky; pine; sap; Asclepias; milkweed; inaugural; PNAS Nexus; open access journal; National Academy of Sciences; >scientist; Max Planck Institute of Colloids and Interfaces (Potsdam, Germany); McGill University (Montreal, Quebec, Canada); mistletoe; viscin; nature; natural; cellulose; cellulosic; skin; wound; sealant; birdlime; adhesion; sticky; chemical substance; tree branch; trap; bird; Roman mythology; mythological; hero; Aeneas; underworld; century; Christmas ornament; Christmas decoration; love; lovers; kiss; archive.org; European mistletoe (Viscum album); parasitic plant; hemiparastic; plant; species; medicine; disease; ailment; Ancient Greece; ancient Greeks; berry; berries; thread (yarn); meter; fiber-reinforced; fitness (biology); evolutionary advantage; seed dispersal; infestation; infest; host (biology); hierarchy; hierarchical; cellulose microfibril; humidity; composite material; matrix; chemical synthesis; synthetic; irreversible process; Matthew Harrington; associate professor; Department of Chemistry at McGill University; Germany; daughter; Christmas; market; stiffness; relative humidity; pascal (unit); GPa; MPa; creep (deformation); flow; deformation (mechanics); strain; chemical process; processing; thin film; 3-D printing; three-dimensional; cube; viscin; thread (yarn); manufacturing; premanufactured; two-dimensional; mesh; heat fusion; fuse; rehydration; Creative Commons Attribution License; hygrometer; hygroresponsive behavior; reversible reaction; mechanical properties; reproducibility; reproducible; contact welding; room temperature; ambient; transparency; transparent; biaxial tensile testing; biaxial stretching; drying; plastics; glass; tissue (biology); biological tissue; skin; cartilage; Nils Horbelt; graduation; graduated; doctor of Philosophy; doctoral student; first author; finger; rubbing; biodegradation; biodegradability; renewable resource; renewability.