Phosphine on Venus
March 8, 2021
Phosphorus is a
chemical element of low
atomic weight (
atomic number = 15). As a consequence, phosphorus is quite
abundant in Earth's crust, existing at about the 0.1% level. Since it sits below
nitrogen in the
periodic table, it has an expected
valence of three. Phosphorus combines with
oxygen to form the
phosphate anion, PO
4-1, that combines with other elements to form many
phosphate minerals.

Pandemonium phosphate, a parody of the many phosphates known to chemists.
(Image © 2015 by the author, but it can be used for personal and educational use. License required for commercial use.
Click for larger image. Other STEM images are here.)
Life on Earth evolved to make use of the available
materials, one of which is phosphorous.
Adenosine triphosphate (ATP, C
10H
16N
5O
13P
3), a
molecule found in all forms of life, is the
energy source and means of energy storage in
cells. ATP is created by
glucose in a process called
glycolysis. The 1997 Nobel Prize in Chemistry was awarded to
Paul D. Boyer (1918-2018),
John E. Walker (b. 1941), and
Jens C. Skou (1918-2018) for discoveries related to ATP.
One phosphorous
compound,
phosphine (PH
3) is uncommon in
nature, since phosphorus is more stable as an
oxide in
oxidizing environments. However, phosphine is a
byproduct of
chemical reactions of life, so its existence has been proposed as a
biosignature for
astrobiology. Natural non-
biological processes for creation of even trace amounts of phosphine are unknown, so detection of phosphine would be indicative of life.
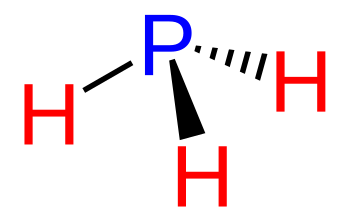
Molecular structure of phosphine (PH3).
(Modified Wikimedia Commons image by NEUROtiker.)
Early in
astronomy, the
planet,
Venus, was viewed as Earth's
twin, since its
radius is nearly identical to that of Earth, smaller by just 5%, and it's
orbital period around the
Sun is 224.7 Earth
days (0.615 Earth
years). For such reasons and because it's closer to the Sun, Venus was depicted in early
science fiction as a
warmer Earth that hosted
tropical forests and often
dinosaurs. As
scientific instrumentation progressed, Venus was found to be quite unlike the Earth.
A day on Venus lasts 243 Earth days. Its
atmosphere is mainly
carbon dioxide (CO
2, 96.5%) with
nitrogen (3.5%) and some
sulfur dioxide (SO
2, 0.015%), and the
atmospheric pressure is a crushing 91
atmospheres. The
surface temperature is 462
°C, 863
°F). Such conditions make the existence of
life on Venus, as we understand life, quite unlikely at its surface. However, it might be possible for
thermoacidophilic extremophile microorganisms, which are known on Earth, to exist in the temperate upper layers of the Venusian atmosphere.

Image of the surface of Venus by the Soviet Venera 9 lander, October 22, 1975. This lander was the first to successfully image the surface of another planet, and it revealed the surface to be composed of non-eroded rocks about 12-16 inches (30 to 40 centimeters) in size. The Soviet Mars 3 lander was the first spacecraft to attain a soft landing on Mars, December 2, 1971. That lander failed after less than two minutes and transmitted no images. The first US Mars lander was the 1997 Mars Pathfinder. (Wikimedia Commons image. Click for larger image.)
In September 2020, a huge international team of
astronomers reported the presence of phosphine in the atmosphere of Venus, sparking a debate on whether this observation was indicative of microbial life on Venus.[1-4]
Research team members were from the
Massachusetts Institute of Technology (Cambridge, Massachusetts),
Cardiff University (Cardiff, UK), the
University of Cambridge (Cambridge, UK), The
University of Manchester (Manchester, UK), the
MRC Laboratory of Molecular Biology (Cambridge, UK),
Kyoto Sangyo University (Kyoto, Japan),
Imperial College London (London, UK), the
Royal Observatory Greenwich (London, UK),
The Open University (Milton Keynes, UK), and the
East Asian Observatory (Hilo, Hawaii).[1]
Spectral detection of a 1.123
millimeter wavelength absorption by a PH
3 rotational transition was done against the bright Venus atmosphere over five
mornings in June 2017.[1] The initial estimate of the phosphine
concentration was 20
parts per billion by volume (ppbv), but further
analysis based on
criticism from another research group forced a revised estimate of 1-4 ppb as a whole planet average, with a peak of 5 to 10 ppb and a 4.8-
sigma confidence level.[1-4] For reference, there is just a global average of a few parts per trillion phosphine in Earth's atmosphere.[4] Such a high level of phosphine cannot be explained by
steady-state chemical and
photochemical processes. There are presently no known ways to produce phosphine in the atmosphere of Venus, at its surface and subsurface, or from
lightning,
volcanoes or by
meteor impacts.[1]
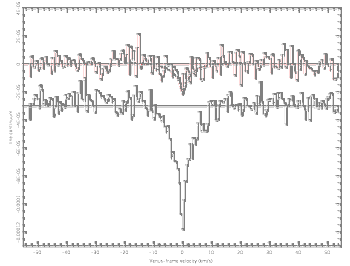
Venus whole-planet absorption spectrum from re-processed Atacama Large Millimeter Array data.
The upper curve is the phosphine rotational transition, and the lower curve is for an HDO transition observed simultaneously and offset for clarity..
(Fig. 3 of Ref. 3, released under a Creative Commons License. Click for larger image.)
Another research group observes that this phosphine feature can be explained by
mesospheric SO
2 at the 100 ppb level[2], and it had further issues with the data analysis.[2] The original research team counters that this level of SO
2 is not realistic.[3] However, the measured spectral line is hidden in a complicated
background signal, and the research team had to
fit the data with a
12th-order polynomial, an extremely messy business.[4]
Composite image of Venus using data from the NASA Magellan spacecraft and NASA Pioneer Venus Orbiter.
Since the surface of Venus is hidden by its dense atmosphere, topography is detected by radar altimetry.
(NASA/JPL-Caltech image. Click for larger image.)
References:
- Jane S. Greaves, Anita M. S. Richards, William Bains, Paul B. Rimmer, Hideo Sagawa, David L. Clements, Sara Seager, Janusz J. Petkowski, Clara Sousa-Silva, Sukrit Ranjan, Emily Drabek-Maunder, Helen J. Fraser, Annabel Cartwright, Ingo Mueller-Wodarg, Zhuchang Zhan, Per Friberg, Iain Coulson, E'lisa Lee, and Jim Hoge, "Phosphine gas in the cloud decks of Venus," Nature Astronomy, September 14, 2020, https://doi.org/10.1038/s41550-020-1174-4, also on arXiv.
- Geronimo Villanueva, Martin Cordiner, Patrick Irwin, Imke de Pater, Bryan Butler, Mark Gurwell, Stefanie Milam, Conor Nixon, Statia Luszcz-Cook, Colin Wilson, Vincent Kofman, Giuliano Liuzzi, Sara Faggi, Thomas Fauchez, Manuela Lippi, Richard Cosentino, Alexander Thelen, Arielle Moullet, Paul Hartogh, Edward Molter, Steve Charnley, Giada Arney, Avi Mandell, Nicolas Biver, Ann Vandaele, Katherine de Kleer, and Ravi Kopparapu, "No phosphine in the atmosphere of Venus," arXiv, October 28, 2020.
- Jane S. Greaves, Anita M. S. Richards, William Bains, Paul B. Rimmer, David L. Clements, Sara Seager, Janusz J. Petkowski, Clara Sousa-Silva, Sukrit Ranjan, and Helen J. Fraser, "Re-analysis of Phosphine in Venus' Clouds," arXiv, December 10, 2020.
- Katherine Wright, "The Venus Phosphine Debate Continues," Physics, vol. 13, no. 201, December 22, 2020.
Linked Keywords: Phosphorus; chemical element; atomic weight; atomic number; abundance of elements in Earth's crust; nitrogen; periodic table; xalence (chemistry); oxygen; phosphate anion; phosphate mineral; Pandemonium Phosphate; parody; phosphate; chemist; STEM image; Life on Earth; material; adenosine triphosphate; molecule; energy; cell (biology); glucose; glycolysis; Paul D. Boyer (1918-2018); John E. Walker (b. 1941); Jens C. Skou (1918-2018); chemical compound; phosphine; nature; phosphorus pentoxide; oxide; oxidizing agent; environment (biophysical); byproduct; chemical reaction; biosignature; astrobiology; biology; biological; molecular structure; Wikimedia Commons; NEUROtiker; astronomy; planet; Venus; twin; radius; orbital period; Sun; day; year; science fiction; temperature; warm; tropical forest; dinosaur; scientific instrument; scientific instrumentation; atmosphere of Venus; atmosphere; carbon dioxide; sulfur dioxide; atmospheric pressure; atmosphere (unit); lithosphere; surface; Celsius; °C; Fahrenheit; °F; life on Venus; thermoacidophilic extremophile; microorganism; Photograph of the surface of Venus by Venera 9; digital image; Soviet Union; Venera 9 lander; erosion; non-eroded; rock (geology); inch; centimeter; Mars 3 lander; spacecraft; Mars; United States; Mars Pathfinder; astronomer; research; Massachusetts Institute of Technology (Cambridge, Massachusetts); Cardiff University (Cardiff, UK); University of Cambridge (Cambridge, UK); University of Manchester (Manchester, UK); MRC Laboratory of Molecular Biology (Cambridge, UK); Kyoto Sangyo University (Kyoto, Japan); Imperial College London (London, UK); Royal Observatory Greenwich (London, UK); The Open University (Milton Keynes, UK); East Asian Observatory (Hilo, Hawaii); absorption spectroscopy; spectral; sensor; detector; millimeter; wavelength; rotational transition; morning; concentration; parts per billion by volume (ppbv); analysis; criticism; standard deviation; sigma; confidence interval; confidence level; chemical equilibrium; steady-state chemical; photochemical; chemical process; lightning; volcano; meteor impact; Atacama Large Millimeter Array; data; curve; semiheavy water; HDO; Creative Commons License; mesosphere; mesospheric; background noise; background signal; polynomial regression; fit; 12th-order polynomial; photomontage; composite image; NASA; Magellan spacecraft; Pioneer Venus Orbiter; density; dense; topography; radar altimetry; JPL-Caltech.