Gap Elements
October 11, 2021
I've visited many
laboratories during my
scientific career as both a
student and a
professional scientist. One feature that nearly all of these had in common was a large
poster of the
periodic table of the elements affixed to a
wall. The
element boxes of the periodic tables in those labs would contain information useful to their particular
field of science.
Chemistry labs would have periodic tables with
atomic weight,
valence, and
density.
Physics laboratories would have information pertaining to their physics specialty, such as
electron binding energy.
2019 marked the 150th
anniversary of the periodic table, and it was
proclaimed the
International Year of the Periodic Table of Chemical Elements (IYPT2019) by the
United Nations General Assembly and
UNESCO. I wrote about that anniversary in an
earlier article (150 Years of the Periodic Table, March 11, 2019). In 1869,
Russian chemist,
Dmitri Mendeleev (1834-1907), organized all the
chemical elements known at that time into a periodic table based on atomic weight.

Dmitri Mendeleev (1834-1907) in an 1887 portrait.
Mendeleev's 1865 doctoral dissertation was entitled "A Discourse on the combination of alcohol and water," and this was the seed of a popular Russian myth that credits Mendeleev with setting the 40% standard strength of vodka. The 40% standard had been set by the Russian government years earlier, in 1843.
As they say, "When all you have is a hammer, everything looks like a nail," so Mendeleev proposed a chemical theory of the luminiferous aether as an inert chemical element weighing less than hydrogen and acting as an all-penetrating, all-pervasive gas.
(Wikimedia Commons image, modified for artistic effect.}
Mendeleev presented his periodic table at a
meeting of the
Russian Chemical Society on March 6, 1869. His paper was entitled, "The Dependence between the Properties of the Atomic Weights of the Elements." This presentation proposed that the chemical elements, when arranged according to their atomic weight, exhibit a
periodicity of properties, such as valence. Based on this periodicity, Mendeleev predicted the existence of three unknown elements,
ekasilicon (germanium),
ekaaluminium (gallium) and
ekaboron (scandium). Germanium was eventually discovered in 1886, gallium in 1875, and scandium in 1879.
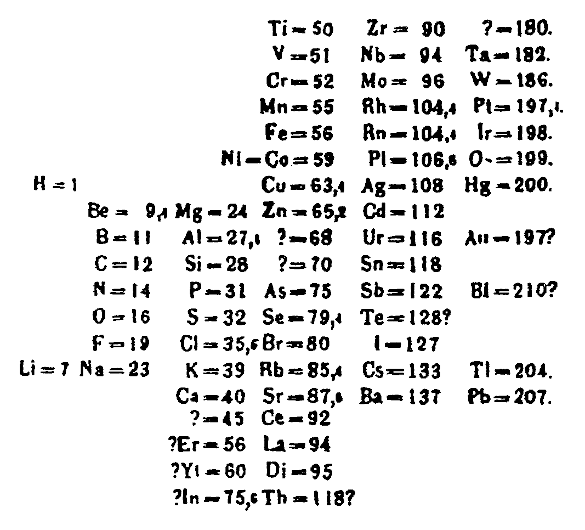
Mendeleev's 1869 periodic table. He published a revised table containing additional elements in 1871.[1] (Wikimedia Commons image. Click for larger image.)
Mendeleev's 1871 periodic table contained a notable
gap between
molybdenum (Mo, element 42) and
ruthenium (Ru, element 44). This element was predicted to have
chemical properties similar to
manganese; and, since it would appear below manganese in the table, it was provisionally named ekamanganese. This element,
technetium (atomic number 43), wasn't discovered until 1937.
Emilio Segrè (1905-1989) and
Carlo Perrier (1886-1948). A discarded piece of molybdenum
foil that had been used as the
deflector in the
Berkeley cyclotron displayed a residual
radioactivity. Segrè and Perrier we able to
chemically separate the
isotopes technetium-95m and
technetium-97 from the molybdenum. The element's name comes from the
Greek word τεχνητός (technitos, "artificial"), since it was the first element to be artificially produced.
Fewer than half of the 118 elements of today's periodic table were known to Mendeleev. The number of elements should hold at 118 for quite some time, since elements of high
atomic number have very short
lifetimes, and they are created only through extreme measures. The next element, the
element with atomic number 119, would be placed below
francium in the periodic table, so it's called
ekafrancium; or, more properly,
ununennium. Both
theory and
experiment have revealed that
synthesis of this element will be much more difficult than that of the previous elements. Element 119, if synthesized, might be the final element accessible with our present
technology.
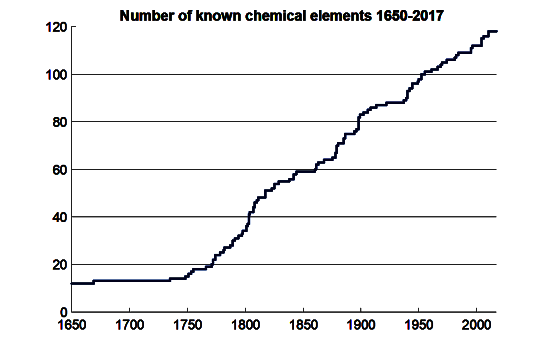
Known chemical elements as a function of time.
Theory indicates that we will stay at a plateau of 118 known elements for a considerable time.
(Wikimedia Commons image. Click for larger image.)
There might be another gap in the periodic table from element 118 and a yet to be observed
element 126 (unbihexium, ekaplutonium). That's because
nuclear shell theory predicts an
island of stability caused by
magic numbers of
protons and
neutrons that lead to
stable nuclear shells.[2]
While lifetimes of isotopes in this gap should be fleeting, lifetimes of isotopes on the island around element 126 may be minutes or days, comparable to the lifetimes of isotopes of elements that populate the lower regions of the periodic table.
unbihexium-310 (an isotope of element 126) is believed to be especially stable, since it's "doubly magic." Its proton number of 126 and neutron number of 184 are both magic numbers.

The nucleon magic numbers, 2, 8, 20, 28, 50, 82 and 126. Isotopes of the chemical elements have been observed to be especially stable when the number of protons, neutrons, or total nucleons, have these values. The anticipated next magic number is 184. (Tarot card base image via Wikimedia Commons. Click for larger image.)
References:
- Dmitri Mendeleev, "Die periodische Gesetzmäßigkeit der Elemente," Annalen der Chemie und Pharmacie, vol. VIII. Supplement (1871), pp. 133-229. An image of Mendeleev's 1871 periodic table can be found at Wikimedia Commons, here.
- Sequence A162626 at The On-Line Encyclopedia of Integer Sequences.
- Neil deGrasse Tyson, "Gaps in the Periodic Table," YouTube video by StarTalk, July 6, 2016.
Linked Keywords: Laboratory; science; scientific; career; student; professional; scientist; poster; periodic table of the elements; wall; chemical element; branches of science; field of science; chemistry; atomic weight; valence (chemistry); density; physics; electron; binding energy; 2019; anniversary; proclamation; proclaimed; United Nations General Assembly; UNESCO; Russia; Russian; chemist; Dmitri Mendeleev (1834-1907); portrait; thesis; doctoral dissertation; alcohol; water; seed; myth; technical standard; concentration; strength; vodka; government; law of the instrument; When all you have is a hammer, everything looks like a nail; theory; luminiferous aether; inert (chemistry); hydrogen; penetration depth; penetrating; gas; Wikimedia Commons; academic conference; meeting; Russian Chemical Society; periodic; periodicity; ekasilicon (germanium); ekaaluminium (gallium); ekaboron (scandium); gap; molybdenum (Mo, element 42); ruthenium (Ru, element 44); chemical property; manganese; technetium; Emilio Segrè (1905-1989); Carlo Perrier (1886-1948); foil (metal); deflection (physics); deflector; Lawrence Radiation Laboratory; Berkeley; cyclotron; radioactive decay; radioactivity; separation process; chemical separation; isotope; technetium-95m; technetium-97; Greek language; Greek word; atomic number; half-life; lifetime; ununennium; element with atomic number 119; francium; ekafrancium; experiment; nucleosynthesis; technology; fFunction (mathematics); time; plateau (mathematics); element 126 (unbihexium, ekaplutonium); >nuclear shell theory; island of stability; magic number (physics); pproton; neutron; stable isotope; unbihexium-310; nucleon magic numbers; Tarot card.