150 Years of the Periodic Table
March 11, 2019
At the
corporate research center where I spent most of my
career, there were many research
laboratories. Some of these were
physics laboratories, and some were
chemistry laboratories. You knew when you were in a physics laboratory by the abundance of
electronic instrumentation, sometimes arranged at the periphery of a large
optical table. The chemistry labs were identifiable mostly by their
odor,
organic labs having a background
solvent smell, and
inorganic labs mostly reeking of
nitrates and
sulfates. There was also the incessant roar of
fume hoods in the chemistry labs. My
materials science laboratory was a composite of these two types with the addition of some
very high temperature furnaces.
Nearly every laboratory, whether physics or chemistry, was sure to have at least one item hanging on the wall - the
periodic table of the elements. While the
element boxes of the periodic tables in the chemistry labs would contain information useful to chemistry, such as
atomic weight,
valence, and
density, the tables in other labs might have such information as the
electron binding energy, which is useful for
x-ray photoelectron spectroscopy, and
crystal structure.
This year, the 150th
anniversary of the periodic table, has been
proclaimed the
International Year of the Periodic Table of Chemical Elements (IYPT2019) by the
United Nations General Assembly and
UNESCO.[1] The Opening Ceremony of IYPT2019 took place at the
UNESCO Headquarters in
Paris, France, on January 29, 2019. It was in 1869 that
Russian chemist,
Dmitri Mendeleev (1834-1907), building on many prior years' work by other chemists, notably
Antoine Lavoisier (1743-1794),
Humphry Davy (1778-1829),
John Dalton (1766-1844), and
Jacob Berzelius (1779-1848), first organized all the chemical elements known at that time into a periodic table based on atomic weight.

Dmitri Mendeleev (1834-1907). Left image, Mendeleev in a 1861 portrait by Sergey Lvovich Levitsky (1819-1898), a few years before his publication of the periodic table; and, right image, Mendeleev in 1897. Both images via Wikimedia Commons, modified for artistic effect.
Since Mendeleev could only be guided in creation of his table by the progression of atomic weights and similarities of
chemical properties such as valence, there were several errors in his original table. The most glaring of these was the inclusion of the supposed element,
didymium, which turned out to be a mixture of the actual elements,
praseodymium and
neodymium. This happened because these elements, members of the so-called
rare earths, are notoriously difficult to
chemically separate.
The discovery of
quantum mechanics finally gave us the principle that defines the similarities and differences between chemical elements. Electrons were found to
populate "shells" of increasing
principal quantum number around
nuclei of different
proton number. These shells have
subshells, designated
s,
p,
d, and
f, that are populated in the following order that gives the periodic table its block shape:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p.
The electron configuration of the first
d-electron metal,
scandium, is
1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d1, 4s2,
while that of the first
f-electron metal,
cerium, is
1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p6, 5s2, 4d10, 5p6, 6s2, 4f1, 5d1, 6s2.
Scientists of all specialties are familiar with the periodic table of the elements. Most of the higher atomic number elements are known to just chemists and materials scientists. Much of my research involved the rare earth elements, so I've personally done experiments with 76 of the elements. One of my wife's inorganic chemistry professors said that he was asked at his dissertation defense to write on a blackboard, from memory, as much of the periodic table as he remembered. (A composite of several Wikimedia Commons images. Click for larger image.)
The February 1, 2019, issue of
Science is a special issue with the theme, "Periodic Table Turns 150,"[2] While the introduction to the issue, "Setting the table," by
Phillip Szuromi is an
open access article,[3] the other articles are unfortunately behind a
paywall for non-members of the
American Association for the Advancement of Science. These include articles entitled,
The quest for superheavies, Ordering the elements, Populating the periodic table: Nucleosynthesis of the elements, Electronic structure in the transition metal block and its implications for light harvesting, and
Rare earth elements: Mendeleev’s bane, modern marvels.
Fortunately, this lack of open access is balanced by a recent open access article on
stellar nucleosynthesis in
Physics Today by
Stan Woosley, a
professor of
astronomy and
astrophysics at the
University of California, Santa Cruz,
Virginia Trimble, a professor of physics and astronomy at the
University of California, Irvine, and
Friedel Thielemann, a
professor emeritus of
theoretical physics at the
University of Basel (Basel, Switzerland).[4] Stellar nucleosynthesis is the process by which chemical elements are created in
stars by their
fusion reactions.
The universe was chemically uninteresting at its creation, since the only elements present after the
temperature had decreased enough to allow the creation of
atomic nuclei were
hydrogen,
helium and
lithium. In 1946,
British astronomer,
Fred Hoyle, proposed the
mechanisms of stellar nucleosynthesis, and this
theory was refined by
Margaret Burbidge,
Geoffrey Burbidge,
William A. Fowler and Hoyle in a 1957
paper.[5] This paper, known by the
shorthand designation,
B2FH, is one of the most
cited astrophysics papers, presently having 1,323
citations in journals published by the
American Physical Society, and 4,276 overall citations as determined by
Google Scholar. Fowler shared the 1983
Nobel Prize in Physics.
Man is always pushing beyond the limits of
nature; so, where no natural element has been found,
synthetic elements have been created. The most interesting example of this is
technetium, Tc, atomic number, 43, synthesized in 1936. Technetium plugged a hole that existed in the periodic table between
molybdenum (Mo, atomic number, 42) and
Ruthenium (Ru, atomic number, 44). While technicium does exist in nature, it has a
half-life of just 4.2 million years, about one-thousandths the
age of the Earth. That means that only one atom of technetium would remain from more than 10
301 atoms that existed at
Earth's formation.
There are other naturally occurring elements that were first known only in their synthesis. These are
promethium,
polonium,
astatine,
francium,
actinium,
protactinium,
neptunium, and
plutonium. There are other elements, those of atomic number 95 and greater, that have such a short lifetime that they are not found in nature and are only obtained through synthesis, as listed in the following table.
As I wrote in an
earlier article (Michael Faraday, October 8, 2018), there's no greater honor for a
scientist than to have an element named after him/her. While some scientists have derived this honor indirectly by having an element named after an institution bearing his name, there are
sixteen elements that have been directly named to honor seventeen scientists.
Curium was named to honor both
Marie Sklodowska-Curie and
Pierre Curie,
einsteinium to honor
Albert Einstein),
fermium to honor
Enrico Fermi,
mendelevium to honor
Dmitri Mendeleev,
rutherfordium to honor
Ernest Rutherford,
copernicium to honor
Nicolaus Copernicus,
seaborgium to honor
Glenn T. Seaborg, and
oganesson to honor
Yuri Oganessian.
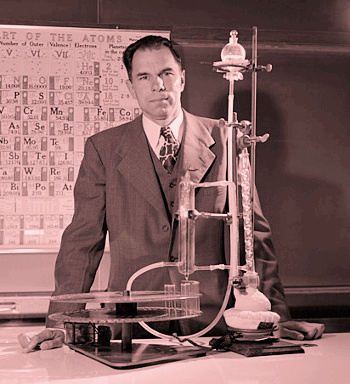
Chemist, Glenn T. Seaborg, in the laboratory.
Seaborg was awarded the 1951 Nobel Prize in Chemistry for his work on the synthesis and identification of ten transuranic elements.
Seaborg died on February 25, 1999, but element-106, Seaborgium (Sg), was named in his honor in 1997. Russian nuclear physicist, Yuri Oganessian, is the only other person to have an element named for him in his lifetime.
Politics is everywhere, even in the sciences, so Seaborg's honor came as a result of a compromise in the naming of elements 104-108.
(Lawrence Berkeley National Laboratory/United States Department of Energy image, via Wikimedia Commons, modified for artistic effect.)
It will be difficult to create and detect elements beyond atomic number 118, since their lifetimes are predicted to be far less than a second. The next element, the
element with atomic number 119, would be placed below
francium in the periodic table, and it's predicted to have chemical properties similar to the other
Group 1 elements, the
alkali metals such as
sodium (Na) and
potassium (K).
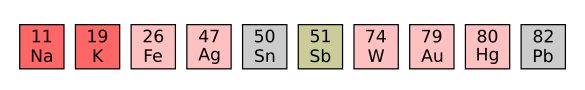
The "oddball" chemical elements. These are the elements whose symbols don't match their English names. The symbol Na for sodium derives from the Latin word for salt, natrium; potassium (K) from the Latin word for potash, kalium; iron (Fe) from its Latin name, ferrum; silver (Ag) from its Latin name, argentum; tin (Sn) from its Latin name, stannum; antimony (Sb) from the Latin name for its mineral, stibium; tungsten (W) from the German name of its mineral, wolframite; gold (Au) from its Latin name, aurum; mercury (Hg) from its Latin and Greek names, hydrargyrum and 'υδραργυρος (hydrargyros, silver water); and, lead (Pb) from its Latin name, plumbum. (Created using Inkscape)
Theodore Gray, one of the
founders of
Wolfram Research, and founder of
Touch Press, a company creating
educational apps that included one on the chemical elements, has built an actual
table with compartments containing specimens of nearly all the elements (the
radioactive and short lifetime ones are absent). Shown below is the
dysprosium compartment of his Periodic Table Table. Dysprosium is used to form the highly
magnetostrictive alloy,
Terfenol-D, Tb
0.3Dy
0.7Fe
2.

Theodore Gray and the dysprosium compartment from his Periodic Table Table. I wrote about Gray in an earlier article (The Periodic Table Table, April 17, 2012). (Left and right images, screen captures from a YouTube video.[6]
References:
- The International Year of the Periodic Table - A Common Language for Science, iypt2019 Website.
- Special Issue - Periodic Table Turns 150, Science, Science, vol. 363, no. 6426 (February 1, 2019).
- Phillip Szuromi, "Setting the table," Science, vol. 363, no. 6426 (February 1, 2019), pp. 464-465, DOI: 10.1126/science.aaw6790.
- Stan Woosley, Virginia Trimble, and Friedel Thielemann, "The origin of the elements," Physics Today, vol. 72, no. 2 (February, 2019), pp. 36-37, https://doi.org/10.1063/PT.3.4134.
- E. Margaret Burbidge, G. R. Burbidge, William A. Fowler, and F. Hoyle, "Synthesis of the Elements in Stars," Rev. Mod. Phys., vol. 29, no. 4 (October 1, 1957), pp. 547ff., DOI:https://doi.org/10.1103/RevModPhys.29.547.
- The Periodic Table Table Featuring Theo Gray, YouTube video by BytesizeScience, February 22, 2012.
- Theodore Gray and Nick Mann, "Elements: A Visual Exploration of Every Known Atom in the Universe," Black Dog & Leventhal Publishers, April 3, 2012, 240 pp., ISBN 978-1579128951 (via Amazon).
Linked Keywords: Corporation; corporate; research; career; laboratory; laboratories; physics; chemistry; electronic; scientific instrument; instrumentation; optical table; odor; organic chemistry; solvent; inorganic chemistry; nitrate; sulfate; fume hood; materials science; muffle furnace; very high temperature furnace; periodic table of the elements; chemical element; atomic weight; valence (chemistry); density; electron; binding energy; x-ray photoelectron spectroscopy; crystal structure; anniversary; proclamation; proclaim; United Nations General Assembly; UNESCO; UNESCO Headquarters; Paris, France; Russia; Russian; chemist; Dmitri Mendeleev (1834-1907); Antoine Lavoisier (1743-1794); Humphry Davy (1778-1829); John Dalton (1766-1844); Jacob Berzelius (1779-1848); portrait; Sergey Lvovich Levitsky (1819-1898); scientific literature; publication; Wikimedia Commons; chemical property; didymium; praseodymium; neodymium; rare earth element; separation process; chemically separate; quantum mechanics; electron shell; principal quantum number; atomic nucleus; nuclei; proton; electron subshell; transition metal; d-electron metal; scandium; lanthanide; f-electron metal; cerium; scientist; branches of science; specialties; chemist; materials science; materials scientist; research; experiment; wife; professor; dissertation defense; blackboard; long-term memory; Science (Journal); Phillip Szuromi; open access article; paywall; American Association for the Advancement of Science; transuranium element; superheavy element; photovoltaic; light harvesting; stellar nucleosynthesis; Physics Today; Stan Woosley; professor; astronomy; astrophysics; University of California, Santa Cruz; Virginia Trimble; University of California, Irvine; Friedel Thielemann; professor emeritus; theoretical physics; University of Basel (Basel, Switzerland); star; nuclear fusion; fusion reaction; temperature; atomic nucleus; atomic nuclei; hydrogen; helium; lithium; British; astronomer; Fred Hoyle; mechanism; theory; Margaret Burbidge; Geoffrey Burbidge; William A. Fowler; scientific literature; paper; shorthand; B2FH paper; B2FH; cite; citation; American Physical Society; Google Scholar; Nobel Prize in Physics; Man; nature; synthetic element; technetium; molybdenum; Ruthenium; half-life; age of the Earth; history of Earth; Earth's formation; promethium; polonium; astatine; francium; actinium; protactinium; neptunium; plutonium; americium; curium; berkelium; californium; einsteinium; fermium; mendelevium; nobelium; lawrencium; rutherfordium; dubnium; seaborgium; bohrium; hassium; meitnerium; darmstadtium; roentgenium; copernicium; nihonium; flerovium; moscovium; livermorium; Tennessine; Oganesson; scientist; sixteen elements that have been directly named to honor seventeen scientists; Marie Sklodowska-Curie; Pierre Curie; Albert Einstein; Enrico Fermi; Dmitri Mendeleev; Ernest Rutherford; Nicolaus Copernicus; Glenn T. Seaborg; Yuri Oganessian; chemist; Nobel Prize in Chemistry; chemical synthesis; transuranic element; element-106, Seaborgium (Sg); nuclear physics; nuclear physicist; politics; science; compromise; Lawrence Berkeley National Laboratory; United States Department of Energy; Wikimedia Commons; Ununennium; element with atomic number 119; francium; Group 1 elements; alkali metal; sodium; potassium; symbol (chemistry); English language; Latin word; halite; salt; potash; iron; silver; tin; antimony; mineral; tungsten; German language; wolframite; gold; mercury (element); Greek language; lead; Inkscape; Theodore Gray; founder; Wolfram Research; Touch Press; education; educational; application software; app; table (furniture); radioactive decay; dysprosium; magnetostrictive; Terfenol-D; YouTube video.