Diamond Elasticity
July 30, 2018
I have a huge collection of
music compact disks (CDs), none of which have been played in the past year. When music CDs were introduced they were much more convenient than
phonograph records, and sales of phonograph records steeply declined. Likewise,
digital music files are much more convenient than CDs, so it's now hard to find music CDs. I think my last music CD purchase was twenty years ago from an actual
brick-and-mortar record store. There are presently no record stores in my area, since nearly all music is procured
online.
One CD in my music collection is
Diamond Music, a 1996 compilation of music by
composer,
Karl Jenkins.[1] This music was used in a series of
diamond gemstone commercials of that time that had the helpful recommendation that a diamond
engagement ring should cost two month's
salary.[2] The sentiment that
Diamonds are Forever was used in those commercials, and this was the title of a much earlier
novel by
Ian Fleming and a
James Bond film.
Chemists know that diamonds are actually perishable and not really
eternal. The "Diamonds are Forever" phrase stems from the popular notion that
diamonds are indestructible. However, diamond is just another
carbon allotrope, so it will
burn. The
Gibbs free energy shows that
oxidation is favored even at
room temperature; viz.,[3]
C(Diamond) + O2(gas) -> CO2(gas)
ΔGf(Diamond) = 0.693 kcal/mole
ΔGf(Oxygen) = 0 kcal/mole
ΔGf(Carbon Dioxide) = -94.258 kcal/mole
ΔG(Reaction) = -94.951 kcal/mole
While the negative energy indicates a favorable
reaction, an
activation energy must be overcome before the reaction initiates. For this reason, diamond will only ignite at a
temperature of 850-1,000
°C in
air, or 720-800 °C in pure
oxygen. In 1772,
Antoine Lavoisier used a
lens to
focus the
Sun's rays onto a diamond in an oxygen atmosphere to heat it sufficiently to produce
carbon dioxide.
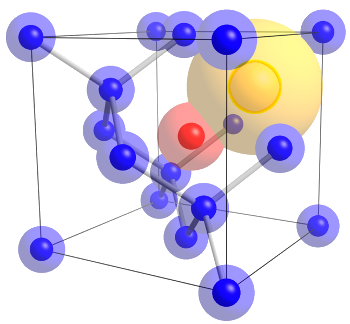
Diamonds are not forever, and they're often imperfect. They can contain atoms other than carbon in their crystal lattice.
This figure shows a nitrogen atom (red) combined with a vacancy (yellow) to form a color center in diamond.
This defect produces a local negative charge that produces a red light photoemission from visible light excitation.
(Wikimedia Commons image by Stacylee14.)
Technology is disrupting many established
markets, from
taxicabs to
television, and it's now disrupting the diamond market. Many of today's young people don't subscribe to the two month's salary diamond, and there are now
synthetic diamond gems available for half the price of a
mined diamond, $4,000 per
carat, rather than $8,000 per carat.[4] Diamond marketer,
De Beers, the "Diamonds are Forever"
corporation, has always argued that synthetic diamonds are somehow inferior to mined diamonds to justify the higher price of mined diamonds.
Seeing how synthetic diamonds are eroding the $80 billion diamond gem market, De Beers has recently announced that it will also supply synthetic diamonds.[4] De Beers is still claiming that synthetic diamonds are inferior, so much so that their synthetics will be sold for about $800 a carat.[4] Many commentators claim that De Beer's sole motivation is to disrupt the synthetic diamond market, as this low price surely will, as well as to underscore the idea of the supposed inferiority of synthetic diamonds.
De Beers says that its synthetics will not be graded for
color and
clarity, as are mined gems, which is apparently another
marketing ploy.[4] Synthetic diamonds are often superior to mined diamonds in such grading, since an
industrial process
can be well controlled and mined diamonds are a product of the
vagaries of
nature. One of my
crystal-growing colleagues was
certified as a diamond
appraiser by the
Gemological Institute of America, possibly as a hedge against diminishing
employment among crystal-growers in the
US.
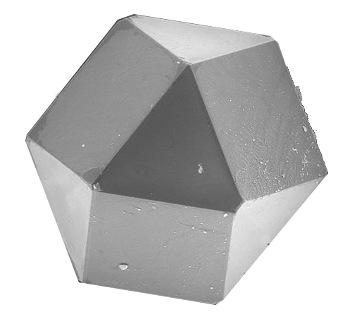
Nature imitating geometry - A scanning electron microscope image of a synthetic diamond cuboctahedron.
A cuboctahedron has 8 triangular faces and 6 square faces. Such a polyhedral shape arises from the different growth rates on different crystal facets.
(Portion of a Wikimedia Commons image by Ludvig14.)
Aside from its use as a gemstone, diamond is a useful
industrial material since it has exceptional
hardness and
strength. The
semi-quantitative Moh's hardness scale that ranks
minerals by which will scratch which, puts diamond at the top with a Moh's hardness value of 10, since it will scratch every other mineral on the list (
talc is the lowest, with a Moh's hardness value of 1). Some of its other
mechanical properties are listed in the following table.[5-6]
The melting point for diamond, of course, applies only in an atmosphere devoid of
oxygen. The tensile strength of diamond could be as high as 90
GPa in the [111]
crystal direction, and 225 GPa in the [100] crystal direction. It should be no surprise that diamond has a very small
compressibility.
While a sheet of
glass will
break when too much
bending force is applied, a
glass fiber that's free of
defects will bend to a very small
bending radius without breaking. This effect is easily seen in the common
thermal insulation material,
Fiberglas/fiberglass. The same is true for
nanocrystal needles of synthetic diamond, as demonstrated by an international
research team from the
City University of Hong Kong (Hong Kong, China), the
Massachusetts Institute of Technology (Cambridge, Massachusetts), the
Institute for Basic Science (Ulsan, Republic of Korea), and the
Nanyang Technological University (Singapore, Republic of Singapore).
Bulk diamond will fracture under applied
stress at a
strain well below one percent.[7,9] The diamond nano-needles will deform with much greater strain, but only when they are mostly free of defects.[8] To prepare such diamond nano-needles, the researchers synthesized diamond thin films on
silicon substrates using
bias-assisted chemical vapor deposition (CVD), and they
plasma etched these films to make the needle structures.[9-10]
The strain experiments were done by pressing the 300
nanometer needles with the
nanoindenter diamond tip of a
scanning electron microscope, where they were found to elastically recover from a strain of about 9%.[7,9-10] This strain is close to the
theoretical limit for diamond, and it corresponds with a tensile strength of up to 98 GPa.[7-8] Needles presumably free of all defects would strain as much as 12% without breaking.[9]
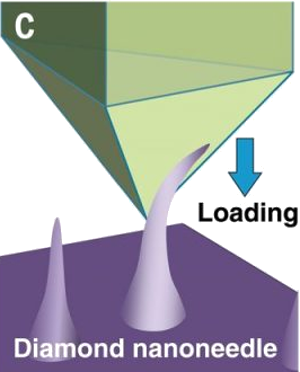
Bending a diamond nanoscale needle in a scanning electron microscope.
(Portion of a Institute for Basic Science (Ulsan, Republic of Korea) image.)
It was found that even
polycrystalline diamond nano-needles could achieve a strain of about half the value, 4%, of crystalline needles.[10-11] The researchers ascribe the high strength of all needles to the small number of defects that can exist in a nanoscale needle and the smooth surfaces of the nano-needles.[7,9] Says
Feng Ding, an
author of this study and a
professor at the Institute for Basic Science, "When outside force is applied to these defects, they can crack and eventually break."[9]
It was necessary to use a
computer model to derive the material stress from the
nonlinear elastic deformation for the diamond needles of the particular shape used in the
experiments.[10] The model showed that the maximum local stress was close to the theoretical tensile strength of defect-free diamond.[10]
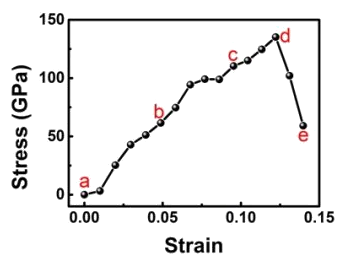
Stress-strain curve for diamond nanoscale needles.
(Portion of a Institute for Basic Science (Ulsan, Republic of Korea) image.)
Not surprisingly, such an unusual material has some potential applications. First, material properties of highly strained materials are often shifted from their
quiescent state. This would allow tuning of such things as
mechanical,
thermal,
optical,
magnetic,
electrical, and
light-emitting properties.[11] In particular, the nitrogen-vacancy emission in diamond, noted in a figure above, should shift in
wavelength.[11] There may be a means of using strain to permanently
encode data in diamonds optically.[11] Since diamond is a
biocompatible material, these nano-needles could be used to deliver
drugs into
cells.[11]
References:
- Karl Jenkins and Adiemus-Palladio 1st Movement from Diamond Music, YouTube Video, January 13, 2009.
- De Beers A Diamond is Forever Commercial (1997), YouTube Video by RetroCommercial.com, March 17, 2011.
- Free energy data from L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- Thomas Biesheuvel, "De Beers to Sell Diamonds Made in a Lab," Bloomberg, May 29, 2018.
- Diamond (C) - Properties, Applications, AZO Material Website.
- R. H. Telling, C. J. Pickard, M. C. Payne, and J. E. Field, "Theoretical Strength and Cleavage of Diamond," Physical Review Letters, vol. 84, no. 22 (May 29, 2000), pp.5160-5163, DOI:https://doi.org/10.1103/PhysRevLett.84.5160.
- Amit Banerjee, Daniel Bernoulli, Hongti Zhang, Muk-Fung Yuen, Jiabin Liu, Jichen Dong, Feng Ding, Jian Lu, Ming Dao, Wenjun Zhang, Yang Lu, and Subra Suresh, "Ultralarge elastic deformation of nanoscale diamond," Science, vol. 360, no. 6386 (April 20, 2018), pp. 300-302, DOI: 10.1126/science.aar4165 .
- Javier LLorca, "On the quest for the strongest materials," Science, vol. 360, no. 6386 (April 20, 2018), pp. 264-265, DOI: 10.1126/science.aat5211.
- UNIST Introduces Novel Method to Grow Elastic Diamonds, UNIST Press Release, April 27, 2018.
- David L. Chandler, "How to bend and stretch a diamond," MIT Press Release, April 19, 2018.
- World's hardest material, diamond, is flexible, Nanyang Technological University Press Release, April 20, 2018.
- Think diamonds are unyielding? Think again, American Association for the Advancement of Science Press Release, April 19, 2018.
- The 4Cs of nanodiamonds, YouTube Video by Nanyang Technological University, April 19, 2018.
Linked Keywords: Music; compact disk; phonograph record; digital audio; digital music file; brick-and-mortar; Internet; online; Diamond Music; composer; Karl Jenkins; diamond; gemstone; television advertisement; commercial; engagement ring; salary; novel; Ian Fleming; Diamonds Are Forever (film); James Bond film; chemist; eternity; eternal; carbon allotrope; combustion; burn; Gibbs free energy; oxide; oxidation; room temperature; kilocalorie per mole; kcal/mole; oxygen; carbon dioxide; negative number; chemical reaction; activation energy; material properties of diamond; ignite; temperature; Celsius">°C; atmosphere of Earth; air; Antoine Lavoisier; lens; focus; Sun's rays; carbon dioxide; atom; crystal structure; crystal lattice; nitrogen-vacancy center; nitrogen; vacancy; F-center; color center; electric charge; negative charge; emission spectrum; photoemission; visible light; excited state; excitation; Wikimedia Commons; Stacylee14; technology; market (economics); taxicab; television; synthetic diamond gem; mining; mined; carat; De Beers; corporation; diamond color; diamond clarity; marketing ploy; industrial process; vagary; vagaries; nature; crystal growth; crystal-growing; colleague; certification; certify; appraiser; Gemological Institute of America; employment; United States; geometry; scanning electron microscope; synthetic diamond; cuboctahedron; triangle; triangular; square; polyhedron; polyhedral; growth rate; crystal; facet; industry; industrial; material; hardness; strength of materials; quantification; semi-quantitative; Moh's hardness scale; mineral; talc; mechanical property; units of measurement; elastic modulus; Young's modulus; pascal unit; GPa; bulk modulus; compressive strength; tensile strength; melting point; oxygen; Miller index; crystal direction; compressibility; glass; fracture; bending stiffness; bending force; glass fiber; crystallographic defect; bending radius; thermal conductivity; thermal insulation; glass wool; Fiberglas/fiberglass; nanocrystal; research; City University of Hong Kong (Hong Kong, China); Massachusetts Institute of Technology (Cambridge, Massachusetts); Institute for Basic Science (Ulsan, Republic of Korea); Nanyang Technological University (Singapore, Republic of Singapore); stress mechanics; deformation mechanics; strain; silicon; wafer; substrate; biasing; bias-assisted; chemical vapor deposition; plasma etching; plasma etch; nanometer; nanoindenter; scanning electron microscope; theory; theoretical; nanoscopic scale; nanoscale; crystallite; polycrystalline; Feng Ding; author; professor; computer simulation; computer model; linearity; nonlinear; experiment; stress-strain curve; quiescent; mechanics; mechanical; temperature; thermal; optics; optical; magnetic; electronics; electrical; light-emitting diode; wavelength; read-only memory; encode data; piocompatibility; biocompatible; pharmaceutical drug; cell.