Self-Assembly
June 12, 2017
Atoms organize themselves into the ordered
crystal structures that we observe because of their inherent tendency to form
chemical bonds. However, this process is tempered by the affect of
atomic size on the possible
geometrical arrangement of the atoms. While
sodium and
cesium are both in the same
group of the
periodic table and have the same +1
ionic valence, they form two different
cubic crystals when combined with the
chlorine -1 valence ion.
That's because cesium (an element with an
ionic radius of 174
pm) is a much larger ion than sodium (ionic radius, 102 pm), and it's closer in size to chlorine (ionic radius, 181 pm). As shown in the figure, the atoms of
sodium chloride (NaCl) arrange themselves into the "
rocksalt" structure in which the sodium and chlorine atoms populate alternating
vertices of a
cube.
Cesium chloride, however, is arranged as two interpenetrating cubic
lattices of cesium and chlorine atoms.
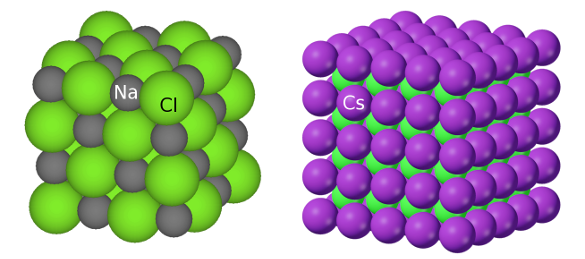
Crystal structures of NaCl and CsCl. The disparate ionic sizes of sodium and cesium cause crystallization in different arrangements. (Left image, and right image, via Wikimedia Commons.)
The importance of atom size in
metallurgy is codified in the
Hume-Rothery_rules rules devised by
English metallurgist,
William Hume-Rothery (1899-1968), in 1926.[1] According to his atomic size rule, which is an
approximation,
metals will enter into
substitutional solid solution if their atomic radii differ by no more than 15%. If their size differs by more than this, there's a likelihood that
secondary phases will form, some of which are detrimental to
alloy strength.
Assembly of
spherical atoms into crystals is a simple case of the more general problem of
self-assembly of
particles. While systems of particles are known to self-assemble,
research on self-assembly has generally been a
one-way street in which self-assembly is analyzed to determine its causes.
Engineering particles to assemble in a selected fashion has been driven mostly by
intuition in constructing the particles, and it's been hit-or-miss whether a particular approach will achieve the desired goal.
In a reaction against this "forward design" approach, a team of
chemical engineers from the
University of Texas at Austin (UTA, Austin, Texas) has been developing an "inverse design" design approach to self-assembly of
colloids.[2-3] As
Thomas Truskett, a UTA
professor and
co-author of the work, explains,
"In a very simplified interpretation, forward design amounts to fabricating particles with novel interactions and then checking to see what they assemble into -- hopefully something desirable... Researchers' physical intuition can help speed the process of realizing desired materials, but this approach is costly from a time perspective and requires some degree of luck or great expense."[3]
Self-assembly of
nanoscale structures would be a cost effective solution for some applications. The interactions between colloidal particles are easily tunable through variation of the material
composition of the particles and their
coating, and composition of the
solvent.[2] Quite a few self-assembled structures have been built from colloids, including a
two-dimensional kagome lattice, as well as simpler structures such as tubes,
micelles,
lamellar sheets, and structures resembling a
capsid virus.[2]
Colloidal interactions can be tuned in a variety of ways; so, how do we select an appropriate interaction to cause self-assembly of a desired structure that we believe will give us a desired
property or
functionality? What is needed is a systematic approach as produced by an
automated inverse design.[2]
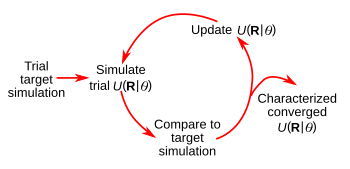
A schematic representation of the iterative inverse design process in which U(R|θ) is the generalized interaction potential for a given particle configuration, R.[2]
(Drawn using Inkscape)
The approach used in the UTA research (see above figure) follows the principles of their 2016 study that used
simulation-based machine learning to optimize the interparticle interactions for a particular self-assembly.[4] The
iterative process varies the interaction
parameters until a desired property is realized.[2]
In their
experiments, the research team discovered some novel pair potentials, including a pair potential that resulted in the self-assembly of a highly
asymmetric,
truncated trihexagonal lattice, and another that produced a
fluid with spherical
voids that they called "
Swiss cheese."[2-3] Interestingly, while the voids ordered into a regular lattice structure, the smaller particles that surrounded the voids were in a disordered, fluidic state.[3]
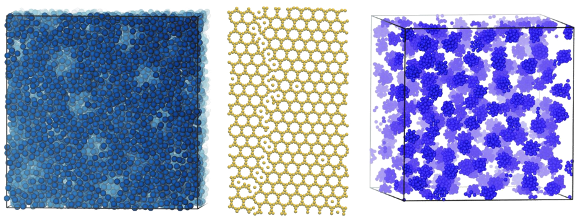
Left, a self-assembled porous mesophase (Swiss cheese); center, a self-assembled truncated hexagonal crystal; right, a self-assembled cluster fluid. (Left and center images by Beth A. Lindquist; right image by Ryan B. Jadrich.)
Says Ryan Jadrich, a
postdoctoral fellow at UTA,
"Inverse design enables the discovery of much more complex materials, on computers, than ever before, and this is a trend we believe will continue... Such tools won't soon replace human researchers, but allow researchers to focus on other, often more interesting tasks that demand creative design. The brunt of the work, which amounts to teasing out subtle details, finding patterns, or performing complex calculations, can now be relegated to automation."[3]
References:
- W. Hume-Rothery, "Research on the nature, properties and conditions of formation of intermetallic compounds, with special reference to certain compounds of tin," J. Inst. Metals., vol 35 (1926), pp. 295-307.
- R. B. Jadrich, B. A. Lindquist, and T. M. Trusketta, "Probabilistic inverse design for self-assembling materials," The Journal of Chemical Physics, vol. 146, no. 18 (2017), Article no. 184103, doi: http://dx.doi.org/10.1063/1.4981796. This is an open access article with a PDF file available here.
- 'Inverse designing' spontaneously self-assembling materials, American Institute of Physics Press Release, May 9, 2017.
- Beth A. Lindquist, Ryan B. Jadrich, and Thomas M. Trusketta, "Communication: Inverse design for self-assembly via on-the-fly optimization," The Journal of Chemical Physics, vol. 145, no. 11 (September 21, 2016), Article no. 111101, doi: http://dx.doi.org/10.1063/1.4962754.
Permanent Link to this article
Linked Keywords: Atom; crystal structure; chemical bond; atomic radius; atomic size; geometry; geometrical; sodium; cesium; group (periodic table); periodic table; ion; ionic; oxidation state; valence; cubic crystal system; chlorine; picometer; pm; sodium chloride; halite; rocksalt; vertex; vertices; cube; cesium chloride; lattice; NaCl; CsCl; crystallization; Wikimedia Commons; metallurgy; Hume-Rothery rules rules; English; metallurgist; William Hume-Rothery (1899-1968); approximation; metal; substitutional solid solution; secondary phase; alloy; strength; sphere; spherical; self-assembly; nanoparticle; particle; research; one-way street; engineering; intuition; chemical engineer; University of Texas at Austin (UTA, Austin, Texas); colloid; Thomas Truskett; professor; co-author; microfabrication; fabricate; physics; physical; material; luck; cost; expense; nanoscopic scale; nanoscale; composition; coating; solvent; two-dimensional; kagome lattice; micelle; lamellar sheet; capsid virus; materials property; functionality; automation; automated; schematic; iteration; iterative; potential energy; Inkscape; computer simulation; machine learning; iteration; iterative; parameter; experiment; asymmetry; asymmetric; truncation; truncate; trihexagonal lattice; fluid; void; Swiss cheese; porosity; porous; mesophase; cluster; postdoctoral fellow; computer; creativity; creative; calculation.