Crystal Alignment Forces
June 1, 2017
There's a saying about trying to put a
square peg into a round hole. As any
geometer will tell you, that's easily accomplished under the constraint that the side of the
square is less than the
circle radius multiplied by the
square root of two (see figure).

Geometry of putting a square peg into a round hole.
In this figure, the circle radius r equals the square side s divided by √2, giving s = (√2)r.
(Created using Inkscape.)
How do we make square holes? As a young
experimenter working on a small budget, I was only able to make square holes by using a small
drill bit to
perforate the outline of the square, punch out the square slug, and then finish the edges with a
file. Eventually, I bought a hole punching tool, manufactured by
Greenlee, to make holes suitable for a common type of one inch square
panel switch.
As you can imagine, making square holes has been a frequent task since the
industrial revolution when a greater quantity of
manufactured items was made. The advent of
electric motors gave us an easy way to make round holes with drill bits, and some
inventors devised methods to drill square holes using a
rotary action. Charles F. Hathaway patented a "Square-Hole Drill" in 1917.[1] In the context of
a picture being worth a thousand words, there's a
silent YouTube video that explains the general concept.[2]
In the
atomic realm, ignoring some constraints that might arise from
chemical bonds, "round peg" atoms will seek to align themselves with low
energy troughs on the surface of
solids. As shown in the figure below of the stacking of atoms on the
(001) face of a
face-centered cubic (FCC)
crystal, atoms on the top layer snuggle into the interatomic areas of the lower layer. If we
cleave an FCC crystal into two pieces and bring them back together, the strongest attractive
force will occur when this same condition is met.
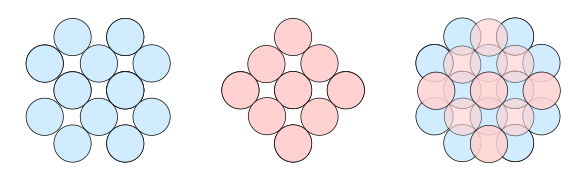
Stacking of atoms on the (001) face of a face-centered cubic crystal. Left, a first layer; middle, a layer on top of the first; and, right, how the layers stack together. (Created using Inkscape.)
Atomic-force microscopy of crystal surfaces has revealed the attractive energy profile of surface atoms, but no direct measurement has been made of how the attractive force between crystal surfaces varies with their
angular alignment. Now, a
research team from the
Pacific Northwest National Laboratory (Richland, Washington) and the
University of Pittsburgh (Pittsburgh, Pennsylvania) has modified an
environmental transmission electron microscope to allow measurement of the
van der Waals forces between the contacting surfaces as a function of crystal alignment.[3-5]
They investigated
rutile, a
compound of
titanium and
oxygen (TiO
2 that exists as a
tetragonal crystal. This was a good object for study, since it's a relatively
hard crystal (
Mohs 6.0-6.5, nearly as hard as
quartz) with good
cleavage. The arrangement of atoms, as shown in the figure, is somewhat more complicated than the FCC example above.

Arrangement of atoms in a rutile unit cell.
The oxygen atoms are colored red, and the titanium atoms are colored gray.
(Wikimedia Commons image by Ben Mills.)
This research gives insights into the process of crystal formation, especially how small crystallites merge to become a larger crystal.[4] Crystallites can fuse seamlessly in attachment to larger crystals when they are are properly
oriented, but what forces attract and align crystal
facets?[4] Says
Kevin Rosso, a PNNL
chemist,
"It's provocative in the sense that from these kinds of measurements one can build a model of 3-D assembly, with particles attaching to each other in select ways like Lego bricks... Crystals are most everywhere in nature, and this work will help us take advantage of these forces when we design new materials.[4]
The research team used an environmental transmission electron microscope equipped with nanocrystal force
sensors to measure the attraction and alignment forces.[4] They attached
nanoscale titanium oxide crystals to opposite sides of their measurement apparatus, changed their angular alignment, and then moved the crystals toward each other until they snapped together.[4] They measured the force it took to pull them apart.[4] The
data showed that the forces were
van der Waals forces.[4-5]
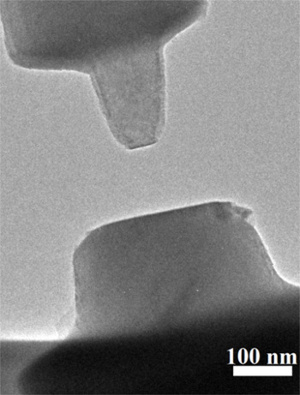
Contacting crystal surfaces of rutile (TiO2), imaged with an environmental transmission electron microscope.
The microscope was modified to allow measurement of the van der Waals forces between the contacting surfaces.
(Xin Zhang/Pacific Northwest National Laboratory image.)
The attraction was weak at tens of
nanometers of separation, and it showed no dependence on alignment. At separations of about one
hydration layer, the attraction became strongly dependent on angular alignment, and it systematically decreases as the
density of the hydration layer increased.[3] The forces agreed with those predicted by the
Lifshitz theory of van der Waals force, a
theory that
models this force on the action of many surface atoms.[3]
As Rosso explains, the fact that the forces are rotationally dependent "...implies that this force will contribute to aligning free crystals that bump together in a
liquid environment, for example, increasing the rate of successful sticking."[4] This research was funded by the
Department of Energy Office of Science.[4]
References:
- Charles F Hathaway, "Square-hole drill," US Patent No. 1,212,634, January 16, 1917.
- How to Drill a Square Hole, YouTube Video by Jill Britton, January 12, 2011.
- Xin Zhang, Yang He, Maria L. Sushko, Jia Liu, Langli Luo, James J. De Yoreo, Scott X. Mao, Chongmin Wang, and Kevin M. Rosso, "Direction-specific van der Waals attraction between rutile TiO2 nanocrystals," Science, vol. 356, no. 6336 (April 28, 2017), pp. 434-437, DOI: 10.1126/science.aah6902.
- For first time, researchers measure forces that align crystals and help them snap together, Pacific Northwest National Laboratory Press Release, April 27, 2017.
- Pulling Apart Titanium Oxide Surfaces, YouTube Video by Pacific Northwest National Laboratory, April 26, 2017.
Permanent Link to this article
Linked Keywords: Square peg in a round hole; geometer; square; circle; radius; multiplication; multiply; square root of two; geometry; Inkscape; experiment; experimenter; drill bit; perforation; perforate; file; Greenlee; control panel; electric switch; Industrial Revolution; manufacturing; manufacture; invention; inventor; rotation; rotary action; A picture is worth a thousand words; silent film; YouTube; video clip; atom; atomic; chemical bond; energy; solid; Miller index; (001); cubic crystal system; face-centered cubic; crystal; cleavage; cleave; force; atomic-force microscopy; angle; angular alignment; research; Pacific Northwest National Laboratory (Richland, Washington); University of Pittsburgh (Pittsburgh, Pennsylvania); environmental transmission electron microscope; van der Waals force; rutile; chemical compound; titanium; oxygen; tetragonal crystal system; hardness; hard; Mohs scale of mineral hardness; quartz; unit cell; Ben Mills; orient; facet; Kevin Rosso; chemist; Lego brick; nature; material; sensor; nanoscopic scale; nanoscale; data; Xin Zhang/Pacific Northwest National Laboratory; nanometer; hydrate; hydration; density; Lifshitz theory of van der Waals force; theory; mathematical model; liquid; environment; United States Department of Energy; Office of Science; Charles F Hathaway, "Square-hole drill," US Patent No. 1,212,634, January 16, 1917.