Shampoo
July 28, 2016
The
proverb, "Cleanliness is next to godliness," is usually attributed to
John Wesley, who included it in a 1778
sermon. His phrasing was slightly different, "Cleanliness is indeed next to godliness," and the meaning of this phrase is usually misinterpreted. The meaning is clear in Wesley's context, "Let it be observed, that
slovenliness is no part of
religion; that neither this, nor any text of
Scripture, condemns neatness of apparel. Certainly this is a duty, not a
sin."
It's been suggested that the necessity of cleanliness arose during the the time of the
Black Death in
mid-14th century. I wrote about the Black Death in a
recent article (Yersinia pestis, February 1, 2016). While the
germ theory of disease wasn't proposed until the
mid-16th century, the earlier
miasma theory, that
vapors from
decomposed matter causes
illnesses, had a long
history.[1]
The
Greek physician,
Hippocrates (c. 460-377 B.C.), thought that bad air caused
pestilence, and the
Roman architect,
M. Vitruvius (c. 75 BC - c. 15 BC), warned of unhealthy vapors from
fetid swamplands.[1]
Galen (c. 130-201 C.E.) extended these bad air
theories by offering a mechanism; namely, that bad air caused an imbalance in
bodily humors.
While
hair can be cleaned with ordinary
soap and
water, the soap will leave a dull
residue without additional
surfactant. When the
British colonized India, they
imported more than just
spices. They also imported a primitive shampoo. The
fruit pulp of
plants of the
genus Sapindus, of which the
lychee is a member, acts as a natural shampoo. These fruits contain
amphipathic glycosides called
saponins that have soap-like properties.
The
German inventor, Hans Schwarzkopf, perfected a
liquid soap for use as shampoo in 1927. In the 1930s, Drene shampoo, the first to contain
synthetic surfactants, was introduced.
Industry was quick to realize the
profit potential of such products, so there was significant
research and development activity in these products in the
mid-20th century. A major milestone was the introduction of
Johnson's Baby Shampoo.

Johnson's Baby Shampoo, advertised as "No More Tears."
The shampoo used amphoteric cleansing agents, which are mild, so they won't sting the eyes. Johnson & Johnson quickly captured a majority of the baby shampoo market.
(From a 1956 issue of Family Circle magazine, via Wikimedia Commons.)
Johnson's Baby Shampoo, which was
advertised as "No More Tears," traded a small measure of
efficacy for mildness. The shampoo used
amphoteric cleansing agents.[2] An amphoteric
compound can act as either an
acid or a
base. For a shampoo, the amphoteric compound is an
ampholyte, a
molecule containing both acidic and basic groups.[2] Being neither acid nor base (or, both acid and base), the
pH of the compound is not that different from that of ordinary water.
The shampoo that I use,
Pert Plus, 2-in-1 Shampoo & Conditioner, works well on my hair; and, I use it also as a surfactant when I develop
novolac-coated
photosensitive printed circuit boards. This shampoo is a thick, green liquid, so there's always the problem of releasing the entire volume from the
container.
This is the problem that
engineers Bharat Bhushan and
Philip Brown from the
Ohio State University Department of Mechanical and Aerospace Engineering solved using surface modification of the shampoo container. Bhushan is a professor of
mechanical engineering, and Brown is a
postdoctoral research associate.
Says Bushan,
"It's what you'd call a first-world problem, right? 'I can't get all of the shampoo to come out of the bottle.' But manufacturers are really interested in this, because they make billions of bottles that end up in the garbage with product still in them."
Shampoos help water to penetrate fabrics, and this property also makes the final drops of liquid cling so tightly to the insides of bottles.[4] While coatings exist that aid in release of consumer items such as ketchup from their bottles, soap-repellent surfaces are a harder problem. Ketchup is mostly water, and water molecules will bond to each other more strongly than to plastic.[4] The "soapy" surfactant molecules in shampoo have low surface tension, so they stick to plastic more readily. Says Brown, "It was an extra challenge for us to make a surface that could repel surfactant."[4]
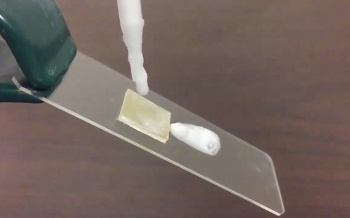
Slip-Sliding Away.
Shampoo running off a treated polypropylene surface.
(Still image from an Ohio State University video by Philip S. Brown.)
One present approach to the problem is to add a
nanoscale texture to the plastic surface, a technique that's expensive, it takes times, and the resulting surface features are fragile.[3-4] The technique discovered by Bushan and Brown is simpler and less expensive than such methods, and it works well for
polypropylene, a common plastic used in
consumer packaging.[4] Their
superoleophobic surfaces are built from
microscopic y-shaped structures composed of
silica nanoparticles.[3-4] These structures cradle the soap
droplets on pockets of
air; so, the shampoo doesn't stick to the plastic, since it never touches the plastic.[4]
Their original process involved
spin-coating the plastic with a
solvent–nanoparticle-polypropylene mixture at an elevated
temperature, then
functionalizing the exposed surface with
fluorosilane to form a durable, super-repellent surface.[3] They further developed the technique so that they could
spray-coat solvent and ultra-fine silica nanoparticles onto the inside of bottles.[4] When the plastic re-hardened, the silica is embedded in the surface.[4] The structures don't cover the surface completely - They're spaced a few
micrometers apart. The texture result in a steep
contact angle for any droplets, so they don't
wet the surface; instead, they form beads and roll right off.[4]
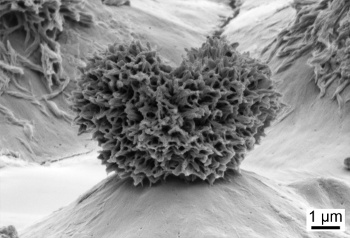
Silica nanoparticles embedded in polycarbonate form.
"Y"-shaped nanoparticles hold the liquid above the plastic surface, thereby preventing sticking.
(Microscope image by Philip S. Brown, Ohio State University.)
177 million
pounds of polypropylene, identified as type-5 in the
Resin Identification Coding System, were used in consumer bottles and bottle lids in the
United States in 2014.[4] It's used, also for
ketchup bottles,
yogurt tubs,
medical bottles,
single-serve coffee pods, and
iced coffee cups.[4] Only about a quarter of these are
recycled, but the recycling process requires a thorough rinsing, a process that's becoming more wasteful in today's water-hungry world. I wrote about the
world's water problems in a
recent article (Future Water Scarcity, March 28, 2016).
There are other uses for such a surface treatment, such as a coating for plastic
biomedical devices, such as
catheters.[4] Bhushan and Brown have applied this technique for coating polycarbonate, which is used for
automobile headlights.[4] Ohio State is looking to
license the technique to manufacturers.[4]
References:
- Carl S. Sterner, "A Brief History of Miasmic Theory," August, 2007 (PDF file).
- Joseph N Masci and Normand A Poirier, "Detergent composition," US Patent No. 2,999,069, September 5, 1961.
- Philip S. Brown and Bharat Bhushan, "Durable superoleophobic polypropylene surfaces," Philosophical Transactions of the Royal Society, vol. 374, no. 2073 (June 27, 2016), DOI: 10.1098/rsta.2016.0193.
- Pam Frost Gorder, "A shampoo bottle that empties completely–every last drop," Ohio State University Press Release, June 26, 2016.
Permanent Link to this article
Linked Keywords: Proverb; John Wesley; sermon; hygiene; slovenliness; religion; scripture; sin; Black Death; mid-14th century; Y. pestis; germ theory of disease; mid-16th century; miasma theory; vapor; decomposition; decomposed; illnesses; history; Ancient Greece; Greek; physician; Hippocrates (c. 460-377 B.C.); infection; pestilence; Roman Empire; architect; M. Vitruvius (c. 75 BC - c. 15 BC); fetid; swampland; Galen (c. 130-201 C.E.); theory; bodily humor; hair; soap; water; residue; surfactant; British; crown colony; colonize; India; import; spice; fruit pulp; plant; genus; Sapindus; lychee; amphiphile; amphipathic; glycoside; saponin; German; inventor; liquid soap; chemical synthesis; synthetic; industry; profit margin; profit potential; research and development; mid-20th century; Johnson's Baby Shampoo; advertising; advertise; amphoterism; amphoteric; eye; Family Circle magazine; Wikimedia Commons; efficacy; chemical compound; acid; base; ampholyte; molecule; pH; Pert Plus, 2-in-1 Shampoo & Conditioner; phenol formaldehyde resin; novolac; photolithography; photosensitive; printed circuit board; container; engineer; Bharat Bhushan; Philip Brown; Ohio State University; Department of Mechanical and Aerospace Engineering; mechanical engineering; postdoctoral research associate; first-world problem; manufacturing; manufacturer; municipal solid waste; garbage; polypropylene; nanoscopic scale; nanoscale; consumer; lipophobicity; superoleophobic; microscopic scale; silicon dioxide; silica; nanoparticle; droplet; air; spin-coating; solvent; temperature; functionalize; fluorosilane; gas dynamic cold spray; spray-coat; micrometer; contact angle; wetting; wet; polycarbonate; pound; resin identification code; Resin Identification Coding System; United States; ketchup; yogurt; medicine; medical; single-serve coffee pod; iced coffee; recycling; recycle; water scarcity; biomedical engineering; biomedical device; catheter; headlamp; automobile headlight; intellectual property; license.