Renal Crystal Growth
September 8, 2016
The
growth of novel
crystals was my main
research occupation for many years. I was involved in the growth of
magnetic garnets for
bubble memory and
magneto-optical devices,
cerium-
doped oxides for use as
xray detectors, and
β-barium borate for
optical frequency doubling. As these crystals were being grown in the
laboratory, I was simultaneously growing a crystal inside my
body - a
kidney stone.

Synthetic Berlinite (AlPO4) crystals grown using a hydrothermal process by some of my colleagues in the 1980s.
This is a centimeter ruler with millimeter divisions.
(My own photo, via Wikimedia Commons.)
According to the
US National Institutes of Health, 8.8% of the
population of the United States has had a kidney stone.[1] Mine was a
side-effect of a
medication I had been taking, and it was
sonically pulverized in situ (or, would that be
in renibus?) by a process called
lithotripsy. I stopped taking that medication, and I've been fine for
decades since.
Although it was not
analyzed, my kidney stone was likely composed of either
calcium oxalate, or
calcium phosphate. Calcium oxalate, which grows when the
urine is too
acidic, is found 80% of the time. Calcium phosphate, which grows when the urine is too
basic, is found 10% of the time.
Astronauts are prone to kidney stones, since
bone loss from inactivity provides the
calcium for kidney stone growth.
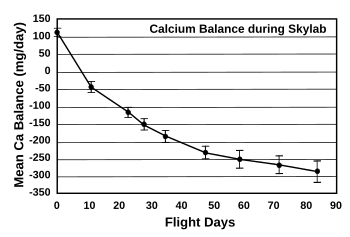
Calcium balance for Skylab astronauts, indicating calcium loss over time.
(Drawn using Inkscape from NASA data in ref. 2.)[2])
When growing crystals from
solution, the degree of
supersaturation is important. Supersaturation is the condition when there is more
material dissolved in a
solvent than the solvent can hold; that is, more than it can
solvate. Supersaturation is easily obtained in nearly all cases by dissolving the material at
high temperature, and then cooling. Most
liquids can solvate greater quantities at high temperature than low.
As an example of
table salt in
water, you can dissolve about 39
grams of
NaCl in a 100 grams of water at 100
°C, but only 35.7 at 20°C. The growth of
rock candy is a good introductory
experiment for
children, and it works well, since 476 grams of
sucrose (
C12H22O11) will dissolve in 100 grams of water at 100°C, but only about 200 gram will dissolve at
room temperature, so you can grow quite a lot of crystal when cooling between those extremes. As usual,
Wikipedia has a convenient
table of solubilities.

Rock candy, crystallized on a stick. In this case, a small amount of yellow food coloring was added to the solution to produce the colored crystals. string or yarn can be used in place of a stick. There are several YouTube videos about the process, including this video. (Photo by Douglas Whitaker (cropped), via Wikimedia Commons.)
As in the kidney stone case, inhibiting crystal formation is often as important as promoting crystal growth. Unwanted crystals can clog
filters in various
industrial processes, and one technique for inhibiting crystal growth is by adding another
chemical to the pot. In one series of
experiments, I found that adding
germanium dioxide inhibited the growth rate of
YAG (yttrium aluminum garnet).[3] In that case, my solution wasn't water, but
molten lead oxide.
For kidney stones,
physicians prescribe potassium citrate. Potassium citrate is an
alkaline chemical that acts as a growth inhibitor for calcium oxalate formation. Such treatment should prevent calcium oxalate kidney stone formation, but what about
dissolution of existing stones? That's the problem that was addressed in
research by
scientists at the
University of Houston (Houston, Texas), the
University of Pittsburgh (Pittsburgh, Pennsylvania), and
Litholink Corporation (Chicago, Illinois).[4-6]

A kidney stone.
One glance at this explains why kidney stones are painful.
The structure also indicates why lithotripsy can easily break these apart.
(University of Houston image.)
As in my YAG experiment and many others, crystal growth inhibitors are merely that - they inhibit crystal growth, but they don't enhance crystal dissolution. The material studied by the researchers,
hydroxycitrate (HCA), actually causes dissolution of calcium oxalate when it's adsorbed onto its crystal surface, and this dissolution occurs even in supersaturated solutions where the hydroxycitrate inhibitor exists in just a tenth percent of the concentration of the calcium oxalate.[4]
Hydroxycitrate is found in
tropical fruits, including the
Malabar tamarind (garcinia cambogia).[6] It's
safe for human consumption, and it's used as a weight-loss
supplement, although such use is not
clinically proven.[7] It's also chemically similar to
citric acid, another chemical shown to be effective as a kidney stone inhibitor.[5]
To uncover the mechanism for this unusual phenomenon, the research team used
atomic force microscopy (AFM) to record, in real time, crystal growth and dissolution at near-
molecular resolution.[5] AFM showed the
facets at the crystal surface shrinking in response to the hydroxycitrate.[5] Their working
hypothesis is that the inhibitor
molecules impart a local
strain to the
crystal lattice, and the crystal reacts to relieve this strain by shedding oxalate and calcium ions.[4-5]
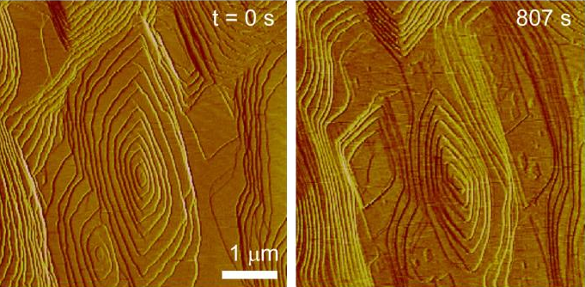
Calcium oxalate monohydrate crystal dissolution, as revealed through atomic force microscopy. (University of Houston image.)
In a clinical study, seven people took a hydroxycitrate supplement for three days. It was found that the hydroxycitrate is
excreted through
urine, which is a requirement if hydroxycitrate is used as a kidney stone treatment.[5] It appears that hydroxycitrate can be used as an alternative treatment to citrate for kidney stones, but a larger clinical study is needed to pinpoint the
dosage and
long-term safety.[4-5]
Says
Jeffrey Rimer,
associate professor of
Chemical Engineering at the University of Houston and an
author of the study, "If it works
in vivo, similar to our trials in the laboratory, HCA has the potential to reduce the incidence rate of people with chronic kidney stone disease."[5]
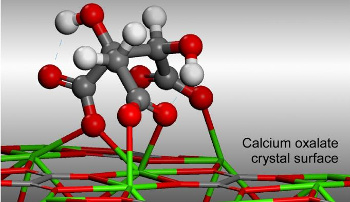
computer modeling indicates that adsorption of hydroxycitrate causes lattice strain that's resolved by crystall dissolution.
(University of Houston image.)
![]()
References:
- Kidney Stones in Adults, National Institutes of Health Web Site.
- Risk of Renal Stone Formation, Report HRP-47060, National Aeronautics and Space Administration, March 2008.
- D.M. Gualtieri, Liquid Phase Epitaxy of Yttrium Aluminum Garnet: Reduction of Growth Rate by Germanium Oxide, Appl. Phys. Lett., vol. 59, no. 6 (August 5, 1991), pp. 650-652.
- Jihae Chung, Ignacio Granja, Michael G. Taylor, Giannis Mpourmpakis, John R. Asplin, and Jeffrey D. Rimer, "Molecular modifiers reveal a mechanism of pathological crystal growth inhibition," Nature, Advanced Online Publication, August 8, 2016, doi:10.1038/nature19062.
- Researchers propose new treatment to prevent kidney stones, University of Houston Press Release, August 8, 2016.
- Jill Daly, "Crystal research finds possible kidney stone treatment," Pittsburgh Post-Gazette, August 16, 2016.
- Steven B. Heymsfield, MD; David B. Allison, PhD; Joseph R. Vasselli, PhD; Angelo Pietrobelli, MD; Debra Greenfield, MS, RD; and Christopher Nunez, MEd, "Garcinia cambogia (Hydroxycitric Acid) as a Potential Antiobesity AgentA Randomized Controlled Trial," JAMA, vol. 280, no. 18 (1998), pp. 1596-1600, doi:10.1001/jama.280.18.1596.
Permanent Link to this article
Linked Keywords: Crystal growth; crystal; research; magnetic; garnet; bubble memory; magneto-optical; cerium; activator; phosphor; doping; oxides; xray; photodetector; β-barium borate; nonlinear optics; optical frequency doubling; laboratory; human body; kidney stone; chemical synthesis; synthetic; Berlinite (AlPO4); hydrothermal synthesis; colleague; 1980s; centimeter; ruler; millimeter; Wikimedia Commons; US National Institutes of Health; demographics of the United States; population of the United States; side-effect; pharmaceutical drug; medication; acoustics; sonic; pulverise; pulverize; in situ; lithotripsy; decade; analysis; analyze; calcium oxalate; calcium phosphate; urine; acid; acidic; base; basic; renal stone formation in space; astronaut; osteoporosis; bone loss; calcium; Skylab; Inkscape; NASA; solution; supersaturation; material; dissolution; dissolve; solvent; solvation; solvate; high temperature; liquid; sodium chloride; table salt; water; grams; NaCl; Celsius; °C; rock candy; experiment; child; children; sucrose; carbon; hydrogen; oxygen; room temperature; Wikipedia; table of solubilities; crystallization; crystallize; food coloring; fiber; string; thread; yarn; YouTube; video; Douglas Whitaker; filtration; filter; industrial process; chemical compound; germanium dioxide; YAG (yttrium aluminum garnet); melting; molten; lead oxide; physician; medical prescription; prescribe; potassium citrate; alkalinity; alkaline; dissolution; research; scientist; University of Houston (Houston, Texas); University of Pittsburgh (Pittsburgh, Pennsylvania); Litholink Corporation (Chicago, Illinois); pain; painful; hydroxycitric acid; hydroxycitrate; tropical fruit; Garcinia gummi-gutta; Malabar tamarind (garcinia cambogia); toxicity; safe for human consumption; dietary supplement">supplement; clinical trial; citric acid; atomic force microscopy; molecule; molecular; image resolution; facet; hypothesis; deformation; strain; crystal structure; crystal lattice; calcium oxalate monohydrate; excretion; excreted; urine; dose; dosage; long-term safety; Jeffrey Rimer; associate professor; Chemical Engineering at the University of Houston; author; in vivo; computer simulation; computer modeling.