High-Entropy Alloys
June 20, 2016
One of the first bits of
chemical knowledge that I learned as a
child was that
oil and
water don't
mix. This was also a common saying at that time that described a variety of
political and
social situations. Oil and water don't mix since water
molecules are
polar, and oil molecules are
non-polar. This results in oil being
hydrophobic. As a
young scientist, however, I was determined to find a process to mix oil and water.
I had among my tools a
vibratory wood saw. This was a
child's version of a
jigsaw built as a powerful
electric buzzer coupled to a jig saw blade. It was relatively
safe, since it would only cut thin pieces of soft wood, such as
balsa. I found that one of my
mother's tall
spice bottles could be inserted in place of the blade to form a vibratory mixer for
liquids.
I placed equal
volumes of
vegetable oil and water into the bottle and mixed them. At first, I thought that I had succeeded, but the mixture
separated after a time back to a layer of oil atop the water. This was my first lesson in
phase separation, a
thermodynamic phenomenon so critical to many chemical processes and the formation of
high strength alloys.
Any quantity of water can be mixed with itself, an
idea that seems trivial in its telling, but an idea that has deeper consequences. How far different can a substance be to still mix with water?
Heavy water (D2O) is somewhat different from H
2O, having a
density of 1.107 g/cc, about 10% greater than that of H
2O. Heavy water is completely
miscible in normal water.
Extending ourselves further,
copper and
nickel are both
transition metals with the
face-centered cubic crystal structure. Nearly all
metals are
miscible in their liquid state, but copper and nickel are so similar that they are completely miscible in the
solid state, as can be seen in their
phase diagram.
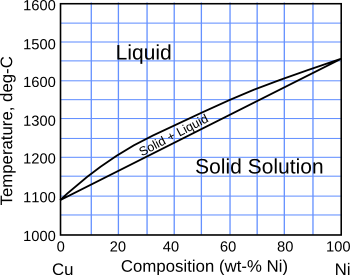
The copper-nickel phase diagram.
(Created from available data using Inkscape.)
Atoms of two-component
solid solutions such as Cu-Ni are in a high
entropy state, since all the
crystal lattice sites of the
alloy are identical, and any one of the two components can reside at any lattice location. Consider the unusual and unlikely case in which nearly equal quantities of many component atoms form a solid solution. The
configurational entropy of such a multi-component alloy would be large; and, surprisingly, such
high entropy alloys exist.
Boltzmann's entropy equation is
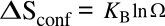
where
KB is
Boltzmann's constant of 1.38065 x 10
23 joule/
kelvin (J/K), and
Ω is the number of
states accessible to a system; that is, the ways in which the
atoms can be differently arranged. In the case of combining atoms of
i different elements of
mole fractions Xi, this equation becomes
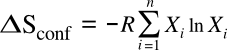
where
R is the
gas constant of 8.314 J/K/
mol. For the special case of equal mixtures of components, we get the configurational entropy values as shown in the graph below.
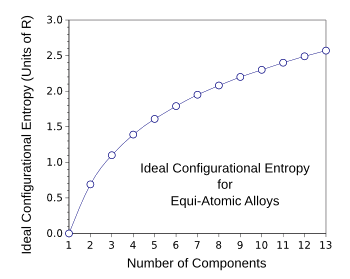
Ideal configurational entropy for equi-atomic multicomponent alloys as a function of the number of components.
(Created with Gnumeric.)
As any
metallurgist knows, liquid mixtures of several different types of metal atoms will give you a solid consisting of multiple crystal types when cooled. This thermodynamic phenomenon has been used to advantage in the creation of high strength alloys, such as those used in
turbine engine blades. For example, MAR-M-247, a
Martin Marietta nickel-based
superalloy has the following composition (
mass-%):[1]
Additionally, a little
hafnium or
zirconium is also added. This alloy has a crystal matrix of the face-centered cubic structure of nickel containing
gamma-prime and carbide phases that provide its high temperature strength.
There was much head-scratching among metallurgists (especially the older,
balding ones) in 2004 when B. Cantor, I.T.H. Chang , P. Knight, and A.J.B. Vincent of the
Oxford University Department of Materials produced a single phase alloy of equal portions of five components.[2] This alloy was
Fe20Cr20Mn20Ni20Co20, which forms a face-centered cubic solid solution. This discovery marked the beginning of serious study on high-entropy alloys.
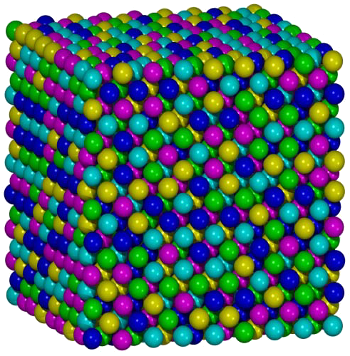
Atomic structure model of the Oxford University FeCrMnNiCo alloy.
In this image, magenta = Fe, green = Co, blue = Cr, cyan = Ni, and yellow = Mn. Aside from slight differences in atomic size, swapping colors would serve just as well, since the composition is equi-atomic.
(Image by Shaoqing Wang, via Wikimedia Commons.)
So, are there
properties of high-entropy alloys that are especially useful? One important application is their potential for use in
nuclear reactors.
Energetic particles such as
neutrons that impinge on an alloy will cause displacement of atoms from their positions in the crystal lattice. This generates
lattice defects and
dislocations that change the alloy's
mechanical properties. Equi-atomic multicomponent alloys have shown good resistance to such
radiation damage.
In an
open access paper in
Physical Review Letters,
scientists from the
University of Helsinki (Helsinki, Finland),
Oak Ridge National Laboratory (Oak Ridge, Tennessee), and the
University of Michigan (Ann Arbor, Michigan), report on
experiments and
modeling that show a substantial reduction of radiation damage in single-phase NiFe and NiCoCr alloys, as compared to
elemental Ni.[3-4]
They ascribe this effect to a reduction in dislocation mobility, with a result that dislocation structures grow more slowly. The radiation damage did not show any phase separation or
amorphization of the alloys.[3] In conventional alloys, a matrix element such as iron exists in greater concentration, and its radiation exposure is proportionately greater. In a high-entropy alloy, the dislodging
probability is distributed among all the
elements.
Scientists from the
Max-Planck-Institut für Eisenforschung (Düsseldorf, Germany) and the
Massachusetts Institute of Technology (Cambridge, Massachusetts) have reported in a recent issue of
Nature a way to transform a high-entropy alloy into a material that exhibits simultaneous
ductility and strength.[5-6]
In conventional alloys, you can have high ductility and low strength, or high strength and low ductility, while high-entropy alloys have high strength, but they're
brittle and not ductile.[6] Their alloy, composed of 50% iron, 30% manganese, 10% cobalt and 10% chromium, has two coexisting crystal structures, and these
transform from one into the other, giving the desired mechanical properties. (see figure).[6]
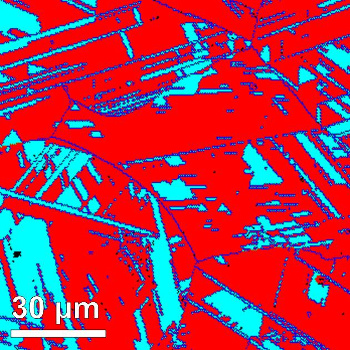
Phases of the Fe50Mn30Co10Cr10 alloy, as imaged using electron backscatter diffraction.
(MPI f. Eisenforschung image, © 2016 Nature.)
![]()
References:
- W. Danesi, J. Hockin, and C. Lund, "Tungsten containing alloy," US Patent No. 3,759,707, September 18, 1973 (via Google Patents).
- B. Cantor, I.T.H. Chang, , P. Knight, and A.J.B. Vincent, "Microstructural development in equiatomic multicomponent alloys," Materials Science and Engineering, vols. 375-377 (July 2004), pp. 213-218, doi:10.1016/j.msea.2003.10.257.
- F. Granberg, K. Nordlund, M. W. Ullah, K. Jin, C. Lu, H. Bei, L. M. Wang, F. Djurabekova, W. J. Weber, and Y. Zhang, "Mechanism of radiation damage reduction in equiatomic multicomponent single phase alloys, Physical Review Letters, vol. 116, no. 13 (April 1, 2016), Document No. 135504. This is an open access paper with a PDF file available here.
- Akshat Rathi, "A new kind of metal could make nuclear reactors stronger and last longer," Quartz, March 21, 2016.
- Zhiming Li, Konda Gokuldoss Pradeep, Yun Deng, Dierk Raabe, and Cemal Cem Tasan, "Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off," Nature (May 18, 2016), doi:10.1038/nature17981.
- Strength and ductility for alloys, Max-Planck-Gesellschaft Press Release, May 24, 2016.
Permanent Link to this article
Linked Keywords: Chemistry; chemical; child; oil; water; mixture; mix; politics; political; society; social; molecule; chemical polarity; polar; non-polar; hydrophobe; hydrophobic; youth; young; scientist; vibration; vibratory; wood; saw; child; jigsaw; electric buzzer; safety; safe; ochroma; balsa; mother; spice; bottle; liquid; volume; vegetable oil; separate; phase; thermodynamic; strength of materials; high strength; alloy; idea; Heavy water (D2O); density; miscibility; miscible; copper; nickel; transition metal; face-centered cubic; crystal structure; metal; solid; phase diagram; Inkscape; solid solution; entropy; crystal lattice; alloy; configurational entropy; high entropy alloy; Boltzmann's entropy equation; Boltzmann's constant; joule; kelvin; microstate; state; atom; mole fractions; gas constant; mole; mol; Gnumeric; metallurgy; metallurgist; turbine engine blades; Martin Marietta; superalloy; mass; tantalum; tungsten; titanium; cobalt; molybdenum; chromium; iron; aluminum; boron; hafnium; zirconium; gamma-prime and carbide phases; hair loss; bald; Oxford University; Department of Materials; space-filling model; Atomic structure model; atomic radius; atomic size; Wikimedia Commons; materials properties; nuclear reactor; energy; energetic; subatomic particle; neutron; crystallographic defect; lattice defect; dislocation; mechanical properties; radiation damage; open access paper; Physical Review Letters; University of Helsinki (Helsinki, Finland); Oak Ridge National Laboratory (Oak Ridge, Tennessee); University of Michigan (Ann Arbor, Michigan); experiment; computer simulation; modeling; chemical element; elemental; amorphous solid; amorphization; probability; Max-Planck-Institut für Eisenforschung (Düsseldorf, Germany); Massachusetts Institute of Technology (Cambridge, Massachusetts); Nature; ductility; brittle; phase transition; transform; electron backscatter diffraction; W. Danesi, J. Hockin, and C. Lund, "Tungsten containing alloy," US Patent No. 3,759,707, September 18, 1973.