Low Density Ice, Shedding Surface Ice
April 4, 2016
Now that the first day of
spring has passed, we anticipate no more
icy weather at
Tikalon's home base in
Northern New Jersey. Spring starts with the passing of the
vernal equinox that occurred this year at 04:30
UT on March 20. This
equinox is the point at which the
Sun crosses from the
southern hemisphere to the
northern hemisphere, and it's a day when night time darkness and day time light are equal.
Daylight increases after that point.
Water is very abundant on the
Earth, and
ice, the
solid phase of water has always interested
scientists. The most interesting property of this
hydrogen-bonded solid is that the
density of ice is less than that of water. Just below
freezing, ice has a density of just 0.9167
g/cc, which is 8.3% less dense than water just above freezing (0.99984 g/cc). The maximum density of water is 0.999973 g/cc at 3.98
°C (see graph).
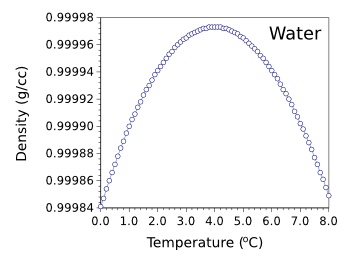
Density of water from 0-8 degrees Celsius.
You can see why 1.0 g/cc works well in most cases.
(Graphed using Gnumeric from available data.)
The density of ice just below freezing is impressively low, and it's the reason that ice
floats on water. This fact must have been important for Earth's
climatic history and the
evolution of life. As if inspired by the phrase, "How low can you go," in the
Chubby Checker song,
Limbo Rock, scientists at the
Dalian University of Technology (Dalian, China), the
University of Nebraska (Lincoln, Nebraska), the
Chinese Academy of Sciences (Beijing, China), and the
University of Science and Technology of China (Hefei, China) have done
calculations that show the possibility of attaining an ice phase with a density of just 0.593 g/cc.[1-2]
The trick to making such an ice is to start first with a
clathrate, a
crystal of mostly water containing a guest
molecules, such as
methane, that stabilizes the structure. It appears that once such a clathrate forms, the guest molecules can be
extracted, leaving just the low density ice behind.[1-2] The structure of the resultant ice crystal is shown below.
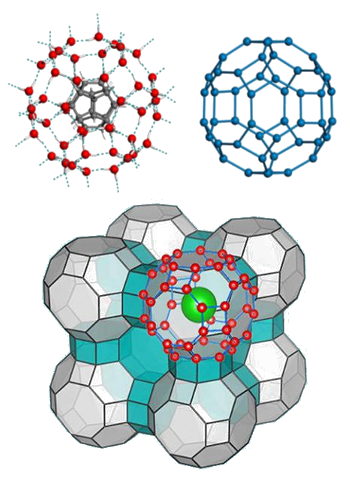
Upper left, a water cage containing a molecule of C20H20, and upper right, the same water cage with the molecule removed.
Lower image, the configuration of an ice crystal built from these units.
(Upper images are from ref. 1, published under a Creative Commons Attribution-NonCommercial license.
Lower image from University of Nebraska-Lincoln/Yingying Huang and Chongqin Zhu)
Says study co-author,
Xiao Chen Zeng,
“Water and ice are forever interesting because they have such relevance to human beings and life... If you think about it, the low density of natural ice protects the water below it; if it were denser, water would freeze from the bottom up, and no living species could survive. So Mother Nature's combination is just so perfect.”[2]
Clathrate compounds can assume various
crystal structures, including
cubic,
hexagonal, and
tetragonal.[1] In previous
research, a guest-free clathrate, now known as
ice XVI, was
synthesized, and this motivated scientists to look for other low density ice clathrates.[1] Clathrates containing methane are found at
high pressure conditions on the
ocean floor, but the contained water structure was not thought to be stable upon removal of the guest molecule.[2]
The research team used a
Monte Carlo packing algorithm and other techniques to predict the cubic clathrate structure, s-III, that has two large
icosihexahedral cavities and six small
decahedral cavities per unit cell.[1] This structure was found to be dynamically stable with the guest molecule removed.[1] Synthesis of the clathrate will be difficult, since it would require a pressure of about four
megabars at -10
°F.[2] The guest molecules could then be extracted by a
vacuum process.[2]
If this ice can be synthesized, it will be the 18th known phase of water; however, it will be known as "Ice XVII," not Ice XVIII. That's not because the ice phases, like elements of a
data array, start at zero. It's because there are two versions of "normal" ice (Ice I), one hexagonal, and the other cubic. The team's research was funded in part by the
National Science Foundation.[1-2]
Ordinary people, including most scientists, are most interested in a specific aspect of ice; namely, how best to remove it from
surfaces, such as
automobile windshields. I've written articles about a method for producing ice-resistant surfaces in an
earlier article (Icing-Resistant Surfaces, August 8, 2012). In that technique, called SLIPS, for "Slippery Liquid Infused Porous Surfaces," a
liquid is held in place on a
nanostructured surface. Since
frost and ice can't adhere to the liquid, it will slide off.[3-4]
Scientists and
engineers from the
University of Michigan (Ann Arbor, Michigan) and the
Air Force Research Laboratory (Edwards Air Force Base, California) have developed a different approach to ice resistance. They found that they could render surfaces ice repellent, or "icephobic," by
coating them with a
clear,
rubbery material that causes ice to quickly slide off because of a
mechanical principal called
interfacial cavitation.[5-8]
Surfaces are classed as icephobic if ice can be removed with a
shear force of ≤ 100
kPa, but passive ice removal in which the ice will just slide off a surface will happen only when the shear force is ≤ 20 kPa.[5] By using a
blend of common
synthetic rubbers, the research team was able to produce coatings with a shear force of ≤ 0.2 kPa, and
durable coatings able to withstand severe
mechanical abrasion,
acid/
base exposure, 100 icing/deicing cycles,
temperature cycling, and
accelerated corrosion.[5-6]
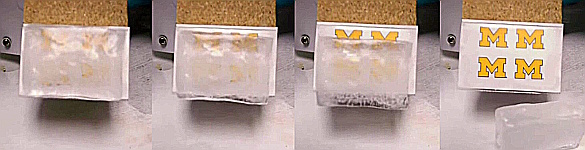
Slip-sliding away. A demonstration of the icephobic coating. The effect is dramatic in this case, since the mass of the thick block of ice generates considerable shear force at the interface. (Still images from a YouTube Video.[8]
As happens often in research, this material property was discovered
by accident.[6] Says
materials science graduate student,
Kevin Golovin,
"Researchers had been trying for years to dial down ice adhesion strength with chemistry, making more and more water-repellent surfaces... We've discovered a new knob to turn, using physics to change the mechanics of how ice breaks free from a surface."[6]
The research team has been able to fine-tune the effect by changing the rubber mix.
Softer coatings, while more ice repellent, are less
durable, and the opposite is true for
harder coatings.[6]
University of Michigan Materials Science and Engineering associate professor,
Anish Tuteja, who led the study, is looking forward to an immediate application.
"I think the first commercial application will be in linings for commercial frozen food packaging, where sticking is often a problem. We'll probably see that within the next year... Using this technology in places like cars and airplanes will be very complex because of the stringent durability and safety requirements, but we're working on it."[6]
This research was funded by the
Office of Naval Research, the
Air Force Office of Scientific Research, and the
National Science Foundation under its
Nanomanufacturing Program.[6]

Real-world demonstration. Keeping your school colors showing in Michigan's icy winter. (University of Michigan image.)
References:
- Yingying Huang, Chongqin Zhu, Lu Wang, Xiaoxiao Cao, Yan Su, Xue Jiang, Sheng Meng, Jijun Zhao, and Xiao Cheng Zeng, "A new phase diagram of water under negative pressure: The rise of the lowest-density clathrate s-III," Science Advances, vol. 2, no. 2 February 12, 2016), Article no. e1501010, DOI: 10.1126/sciadv.1501010. This is an open access publication with a PDF file available here.
- Scott Schrage, "Scientists reveal new ice with record-low density," University of Nebraska-Lincoln Press Release, February 12, 2016.
- Philseok Kim, Tak-Sing Wong, Jack Alvarenga, Michael J. Kreder, Wilmer E. Adorno-Martinez and Joanna Aizenberg, "Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance," ACS Nano, Article ASAP, June 10, 2012, DOI: 10.1021/nn302310q
- Michael Patrick Rutter and Twig Mowatt, "A new spin on antifreeze - Researchers create ultra slippery anti-ice and anti-frost surfaces," Harvard School of Engineering and Applied Sciences Press Release, June 11, 2012.
- Kevin Golovin, Sai P. R. Kobaku, Duck Hyun Lee, Edward T. DiLoreto, Joseph M. Mabry and Anish Tuteja, "Designing durable icephobic surfaces," Science Advances, vol. 2, no. 3 (March 11, 2016), Article no. e1501496, DOI: 10.1126/sciadv.1501496. This is an open access publication with a PDF file available here.
- Gabe Cherry, "Spray-on coating could ice-proof airplanes, power lines, windshields," University of Michigan Press Release, March 11, 2016.
- Ben Thompson, "How scientists developed the most effective ice-proofer yet," Christian Science Monitor,March 13, 2016.
- University of Michigan Icephobic Coating, YouTube Video, March 9, 2016.
Permanent Link to this article
Linked Keywords: Spring; ice; icy; weather; Tikalon; Morris County, New Jersey; Northern New Jersey; vernal equinox; Universal Time; UT; equinox; Sun; southern hemisphere; northern hemisphere; daylight; water; Earth; solid; phase; scientist; hydrogen bond; hydrogen-bonded; density; freezing; kilogram per cubic meter; g/cc; Celsius; °C; Gnumeric; buoyancy; float; paleoclimatology; climatic history; evolution of life; Chubby Checker; Limbo Rock; Dalian University of Technology (Dalian, China); University of Nebraska (Lincoln, Nebraska); Chinese Academy of Sciences (Beijing, China); University of Science and Technology of China (Hefei, China); calculation; clathrate hydrate; crystal; molecule; methane; extraction; extracted; molecule; dodecahedrane; C20H20; Creative Commons Attribution-NonCommercial license; Xiao Chen Zeng; human beings; species; Mother Nature; crystal structure; cubic crystal system; hexagonal crystal system; tetragonal crystal system; research; ice XVI; chemical synthesis; synthesize; high pressure; seabed; ocean floor; Monte Carlo method; packing; algorithm; icosihexahedral; decahedral; megabar; Fahrenheit; °F; vacuum; array data structure; data array; National Science Foundation; ordinary people; surface; automobile; windshield; liquid; nanostructure; frost; engineer; University of Michigan (Ann Arbor, Michigan); Air Force Research Laboratory (Edwards Air Force Base, California); coating; transparent; clear; rubbery; material; classical mechanics; mechanical; interface; interfacial; cavitation; shear stress; shear force; pascal; kPa; polymer blend; synthetic rubber; toughness; durable; mechanical abrasion; acid; base; temperature cycling; accelerated life testing; corrosion; mass; YouTube Video; serendipity; accident; materials science; postgraduate education; graduate student; Kevin Golovin; chemistry; physics; hardness; hard; University of Michigan Materials Science and Engineering; associate professor; Anish Tuteja; commercial; frozen food; packaging; technology; car; airplane; safety; Office of Naval Research; Air Force Office of Scientific Research; National Science Foundation; Nanomanufacturing Program; school colors; Michigan; winter.