SnIP - An Inorganic Double Helix
October 27, 2016
Sulfur is an ubiquitous
element on
Earth, since it forms
compounds with many other elements. One example is FeS
2,
iron pyrite, also called
fool's gold because of its
golden luster. Another example is PbS,
lead(II) sulphide, that's found in
mineral form as
galena. Lead sulfide was used as a
"cat's whisker" detector in
crystal radio sets to form a
point-contact diode, and it's an excellent
infrared photoconductor.

A "cat's whisker" galena radio detector. Certain crystal facets are better for this application, so the whisker is moved across the mineral to find a "sweet spot."
(Photo by J.A. Davidson, via Wikimedia Commons.)
Sulfur is interesting among
inorganic chemicals in its tendency to form
rings and polymeric chains. Rings of eight sulfur
atoms exist in
liquid sulfur; and, by
Heating to high
temperature and
quenching to
room temperature, a sulfur
polymer can be formed (see figure).
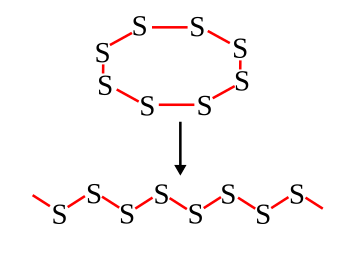
It's an easy process to transition from an S8 ring to a polymer chain.
Heating breaks the S8 rings, and these combine into chains.
(Created using Inkscape.)
There's a lot of interesting
science behind this polymeric transition. While sulfur melts to a
low-viscosity light yellow liquid at about 120
°C, its
viscosity increases by four
orders of magnitude in the temperature range of 159°C to 184°C.[1] At these high temperatures, there is an
average of a million sulfur atoms in polymer chains.[1] The simplicity of this process is demonstrated in a
YouTube video.[2]
Sulfur is interesting for another reason. Although known for its
rotten egg odor, it has a connection to the
rose in
Juliet's famous speech in
Shakespeare's Romeo and Juliet,
What's in a name? that which we call a rose
By any other name would smell as sweet;
When I was a
young student of
chemistry, sulfur was known also as sulphur, the first being the more common
American usage, and the later being the common
British English usage. I still
type sulphur, which is how I learned it from the chemistry
textbook my
father had in his
G.I. Bill college days, and my
spell checker corrects me. The
International Union of Pure and Applied Chemistry adopted the sulfur spelling in 1990.[3] The
Royal Society of Chemistry switched from sulphur to sulfur in its
publications in 1992, although there's still considerable
controversy about the change.[4] Still, sulfur by any other name...
Inorganic polymers are rare (
aluminum phosphate, AlPO
4, will also form polymer chains in solution), but another interesting member has been added to the list. A team of
German scientists led by members at the
Technical University of Munich has discovered a double
helix inorganic
material, SnIP, that's a
semiconductor with interesting
optical and
electronic properties.[5-7] Since the
synthesized form is a
fiber, it also has
mechanical flexibility.[5-6]
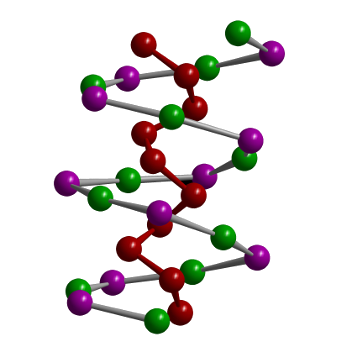
The double helix structure of SnIP.
Although it's not clear from the references, my conjecture is that the red atoms are phosphorus, the green atoms are tin, and the violet atoms are iodine.
(Technical University of Munich image by Prof. Tom Nilges.)
This is an exciting discovery, since SnIP is comprised of the
abundant and inexpensive elements,
tin,
iodine, and
phosphorus. The
research team has been able to synthesize
gram quantities of SnIP, which functions as a semiconductor much like
gallium arsenide, but without the
toxicity.[6-7]
Centimeter-length fibers of SnIP have been produced, and they're extremely flexible. These fibers can be separated into
nano-sized fibers of about 20
nanometer size comprised of just a few double helix strands.[6-7]
A
racemic mixture of
right- and
left handed double helices is produced in just minutes by the demonstrated synthesis process.[5] The SnIP double helices can be
suspended in
solvents such as
toluene to produce thin layers by solvent
evaporation.[6] SnIP is stable up to about 500°C (930°F), so it could be used in
concentrator solar cells.[6-7] SnIP has a
band gap of 1.86
eV,[5] so it's slightly more responsive to infrared light than gallium arsenide, which has a band gap of 1.43 eV.
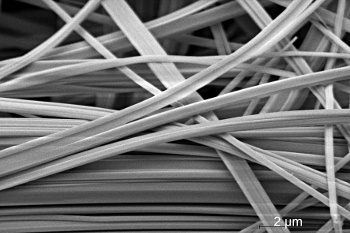
A scanning electron microscope image of SnIP fibers.
(Max Planck Institute for Solid State Research, Stuttgart, image by Viola Duppel.)
Since SnIP could be
doped with other elements, it might be used as a
photocatalyst and a
thermoelectric material.[6]
Theoretical calculations indicate that quite a few doping elements are possible. Since SnIP helices are formed in both right- and left-handed forms, this might lead to some interesting
optoelectronic applications.[6] This research was funded in part by the German Research Foundation (
Deutsche Forschungsgemeinschaft).[5]
References:
- V.F. Kozhevnikov, W.B. Payne, J.K. Olson, C.L. McDonald, and C.E. Inglefield, "Physical Properties of Sulfur Near the Polymerization Transition," arXiv, May 3, 2004.
- Hardware Science, "Sulfur Polymer," YouTube Video, February 2, 2015.
- Editorial - So long sulphur, Nature Chemistry, vol. 1, no. 5 (August, 2009), p. 333, doi:10.1038/nchem.301.
- Colin Cook, "Return of sulphur," Royal Society of Chemistry, May 22, 2012.
- Daniela Pfister, Konrad Schäfer, Claudia Ott, Birgit Gerke, Rainer Pöttgen, Oliver Janka, Maximilian Baumgartner, Anastasia Efimova, Andrea Hohmann, Peer Schmidt, Sabarinathan Venkatachalam, Leo van Wüllen, Ulrich Schürmann, Lorenz Kienle, Viola Duppel, Eric Parzinger, Bastian Miller, Jonathan Becker, Alexander Holleitner, Richard Weihrich and Tom Nilges, "Inorganic Double Helices in Semiconducting SnIP," Advanced Materials, September 14, 2016, DOI: 10.1002/adma.201603135.
- Inorganic double helix, Technical University of Munich Press Release, August 26, 2016.
- Dexter Johnson, "Novel Semiconductor Has Double-Helix Structure of DNA," IEEE Spectrum, September 13, 2016.
- Animation of the SnIP double helix structure, Technical University of Munich YouTube video, September 9, 2016.
- Technical University of Munich video of the bending of a SnIP needle.
Permanent Link to this article
Linked Keywords: Sulfur; sulphur; chemical element; Earth; chemical compound; iron pyrite; golden; luster; lead(II) sulphide; mineral; galena; cat's whisker detector; crystal radio set; point-contact diode; infrared; photoconductivity; photoconductor; radio; detector; crystal; facet; J.A. Davidson; Wikimedia Commons; inorganic compound; inorganic chemical; allotropes of sulfur; ring; polymeric chain; atom; liquid; heating; temperature; quench; room temperature; polymer; polymer chain; Inkscape; science; viscosity; Celsius; orders of magnitude; average; YouTube; video; hydrogen sulfide; rotten egg odor; rose<; Juliet; A rose by any other name would smell as sweet; William Shakespeare; Romeo and Juliet; middle school; young student; chemistry; American; British English; computer keyboard; type; textbook; father; G.I. Bill; college; spell checker; International Union of Pure and Applied Chemistry; Royal Society of Chemistry; scientific literature; publication; controversy; aluminum phosphate; German; scientists; Technical University of Munich; helix; material; semiconductor; optics; optical; electronic; chemical synthesis; synthesize; fiber; classical mechanics; mechanical; deflection; flexibility; molecular structure; conjecture; phosphorus; tin; iodine; Tom Nilges; abundance of the chemical elements; research; gram; gallium arsenide; toxic; toxicity; centimeter; nanoscopic scale; nano-size; nanometer; racemic mixture; right-hand rule; Left-hand rule; suspension; suspended; solvent; toluene; evaporation; concentrator photovoltaics; solar cell; band gap; electronvolt; eV; scanning electron microscope; micrograph; image; Max Planck Institute for Solid State Research, Stuttgart; Viola Duppel; doping of semiconductors; dope; photocatalysis; photocatalyst; thermoelectric effect; thermoelectric; theory; theoretical; calculation; optoelectronic; Deutsche Forschungsgemeinschaft.