Sponges of Boron Nitride
January 28, 2016
One of my first
scientific papers was published in the now defunct journal, High Temperature Science.[1] High Temperature Science, published from 1969-1991, was founded and
edited by the prolific
chemist,
John L. Margrave.[2] Margrave, who was elected to the
National Academy of Sciences in 1974, received his first degree in
physics from the
University of Kansas in 1948 after serving in the
US Army Signal Corps during
World War II.[2]
Margrave discovered that he was more interested in
chemistry, so he continued his
graduate education in chemistry at Kansas. Completing
experiments in
fluorine chemistry under high
temperature and high
pressure conditions, Margrave wrote a 300 page
dissertation and completed his
Ph.D. in just two years. After that, he worked as an
Atomic Energy Commission postdoctoral fellow under
Leo Brewer at the
University of California, Berkeley.[2] Brewer and
Kenneth Pitzer were
co-authors of the revision of a popular
thermodynamics textbook authored by
Gilbert N. Lewis and
Merle Randall.[3]
Margrave became an instructor in the chemistry department at the
University of Wisconsin-Madison in 1952, and much of his
research involved fluorine
bomb calorimetry.[2] In this technique, a useful complement to
oxygen bomb calorimetry, a substance is burned in a fluorine atmosphere so that its
heat of formation can be calculated. Margrave moved to
Rice University in 1963, a relocation perhaps facilitated by Kenneth Pitzer, who was the university
president at the time.

While I was doing differential scanning calorimetry as a graduate student, a colleague of mine was doing fluorine bomb calorimetry in an apparatus much like this.
(Photo by Akshat Goel, via Wikimedia Commons.)
One principal theme of Margrave's research at Rice was
fluorination of
carbon compounds, principally the fluorination of
graphite to form "white graphite." This fluorinated graphite compound, known as
CFX, is an excellent
solid lubricant in
oxidizing and
corrosive environments at high temperature.[2] One of Margrave's students told me that they had fluorinated
peanut butter to see what would happen. Perhaps this was one of those unsanctioned experiments that students are known to do. Interestingly, Margrave was
co-inventor of an improved
embalming fluid.[4]
Boron nitride is another high temperature solid lubricant, since its
hexagonal polymorph has a layered structure like that of graphite. It's stable up to about 800
°C in
air, but only because its oxidation product,
boric oxide, forms a protective surface layer. As the calculated
free energy of the oxidation reaction shows, the oxidation to boric oxide is preferred in oxygen at all temperatures.[5] However, boron nitride is stable in
inert atmospheres,
vacuum, and
reducing atmospheres, up to about 2000°C.
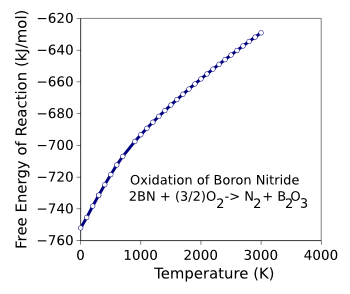
Boron nitride is not as stable as its oxide, as the calculated free energy of the boron nitride oxidation reaction shows. It's stable in air only because boric oxide forms a protective coating.
(Graphed using Gnumeric from NIST data.[5]
Hexagonal boron nitride has another use.
Scientists at
Deakin University (Victoria, Australia),
Drexel University (Philadelphia, Pennsylvania), and the
Missouri University of Science & Technology (Rolla, Missouri) have created an
aerogel of boron nitride that functions as a
sponge for
oil.[6-9] The aerogel is formed from boron nitride
nanosheets.[6-9]
The boron nitride nanosheets, composed of just a few layers of hexagonal boron nitride, have a pore structure that allows absorption of more than 33 times their
weight in
organic solvents, such as oil.[6,8] The aerogels have a
density of just 1.4
mg/
cc, about 1,500 times less than the density of bulk boron nitride. These aerogels are formed as freestanding
membranes by
freeze-drying.[6] The
surface area per
gram is about the area of five and a half
tennis courts.[8-9]

Left, a freestanding boron nitride membrane. Right, a Scanning electron micrograph of the boron nitride nanosheet pore structure. left image via Deakin University; right image via Drexel University)
The
2010 Gulf Coast oil spill had a reported cost of $40 billion.[9] Says Deakin University
professor and an
author of the
paper describing this research,
Ying Chen,
"Oil spills are a global problem and wreak havoc on our aquatic ecosystems, not to mention cost billions of dollars in damage... Everyone remembers the Gulf Coast disaster, but here in Australia they are a regular problem, and not just in our waters. Oil spills from trucks and other vehicles can close freeways for an entire day, again amounting to large economic losses... We are so excited to have finally got to this stage after two years of trying to work out how to turn what we knew was a good material into something that could be practically used."[8]
The research team developed an oil-absorbing boron nitride
powder in 2013, but a powder form is not suitable for oil
remediation. Says Deakin University's
Weiwei Lei, another member of the research team, "...you cannot simply throw powder onto oil – you need to be able to bind that powder into a sponge so that we can soak the oil up, and also separate it from water."[8] A one-step mechano-chemical process was used to
exfoliate the nanosheets from the hexagonal boron nitride and
functionalize them with
amino groups to create highly
water-dispersible particles.[6]
As another interesting
material property, the nanosheets show a strong blue
light emission under exposure to
ultraviolet light, in both their dispersed and dry states.[6] This research was supported by the
Australian Research Council.[9]
References:
- D.M. Gualtieri and P.J. Ficalora, Electron Transfer and Metallic Bonding: The Heats of Reaction of FeAl3-x(Ag;Zn;Pt;Au)x Alloys, High Temperature Science, vol. 7, pp. 25-36 (1975).
- James L. Kinsey, "Biographical Memoir-John Margrave, National Academy of Sciences, 2014.
- Gilbert Newton Lewis and Merle Randall, "Thermodynamics," Kenneth S. Pitzer and Leo Brewer, Eds., McGraw Hill (Second edition, 1961), 723 pp. (Amazon).
- James W. Campbell and John L. Margrave, "Embalming composition and method," US Patent No. 5,405,606, April 11, 1995 (Google Patents).
- NIST-JANAF Thermochemical Tables, US National Institute of Standards and Technology Web Site.
- Weiwei Lei, Vadym N. Mochalin, Dan Liu, Si Qin, Yury Gogotsi, and Ying Chen, "Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization," Nature Communications, vol. 6, article no. 8849 (November 27, 2015), doi:10.1038/ncomms9849. This is an open access publication with a PDF file available here.
- Supplementary figures for ref. 6 .
- Drexel Materials Scientists Aid Australian Institution in Developing Super-Absorbent Material That Can Soak Up Oil Spills, Drexel University Press Release, November 30, 2015.
- Deakin scientists create revolutionary material to clean oil spills, Deakin University Press Release, November 30, 2015
Permanent Link to this article
Linked Keywords: Scientific literature; scientific paper; journal; editor; chemist; John L. Margrave; National Academy of Sciences; physics; University of Kansas; US Army Signal Corps; World War II; chemistry; graduate school; graduate education; experiment; fluorine; temperature; pressure; thesis; dissertation; Doctor of Philosophy; Ph.D.; United States Atomic Energy Commission; postdoctoral research; postdoctoral fellow; Leo Brewer; University of California, Berkeley; Kenneth Pitzer; author; thermodynamics; textbook; Gilbert N. Lewis; Merle Randall; University of Wisconsin-Madison; research; bomb calorimetry; oxygen; standard enthalpy of formation; heat of formation; Rice University; president; differential scanning calorimetry; fluorine; Wikimedia Commons; halogenation; fluorination; carbon compound; graphite; dry lubricant; solid lubricant; oxide; oxidize; corrosive; environment; peanut butter; invention; inventor; embalming chemical; embalming fluid; boron nitride; hexagonal crystal system; polymorphism; polymorph; celsius; air; boron trioxide; boric oxide; Gibbs free energy; inert gas; inert atmosphere; vacuum; reducing atmosphere; chemical stability; chemical reaction; Gnumeric; National Institute of Standards and Technology; NIST; scientist; Deakin University (Victoria, Australia); Drexel University (Philadelphia, Pennsylvania); Missouri University of Science & Technology (Rolla, Missouri); aerogel; sponge; petroleum; oil; nanosheet; weight; organic solvent; density; gram; mg; cubic centimeter; cc; membrane; freeze-dry; surface area; tennis court; scanning electron micrograph; Deepwater Horizon oil spill; 2010 Gulf Coast oil spill; professor; academic publishing; paper; Ying Chen; oil spill; aquatic ecosystem; Australia; waterway; truck; vehicle; controlled-access highway; freeway; economy; economic; material; powder; environmental remediation; Weiwei Lei; intercalation; exfoliation; exfoliate; functional group; functionalize; amine; amino group; dispersion; water-dispersible; material property; light emission; ultraviolet ligh; Australian Research Council; Gilbert Newton Lewis and Merle Randall, "Thermodynamics," Kenneth S. Pitzer and Leo Brewer, Eds., McGraw Hill (Second edition, 1961), 723 pp.; James W. Campbell and John L. Margrave, "Embalming composition and method," US Patent No. 5,405,606, April 11, 1995.