Building Metallic Gold
May 11, 2015
Atoms at the
surfaces of
crystals are different from atoms in their interior. From a
chemical standpoint, surface atoms have "
dangling bonds;" that is,
atomic orbitals that would have
bonded with absent surrounding atoms. The different
electron states of these orbitals are responsible for the
catalytic properties of surfaces.
As shown in the figure, there are more dangling bond at some surface features of a crystal, such as steps, and this explains the enhanced catalytic activity of some
materials. This is just one feature of a good
catalyst, since other factors are involved, such as the material's resistance to
reacting with the
precursor chemicals of the catalytic
reaction.[1] That's the principal reason why
platinum and
rhodium are such excellent catalysts.
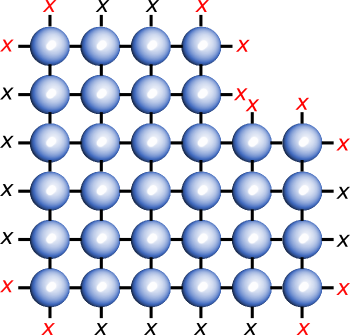
A crystal plane showing dangling bonds.
Some features on a crystal surface - steps, kinks, vacancies, and adatoms[1] - have a higher concentration of dangling bonds.
(Illustration by the author using Inkscape.)
A
nanocrystal has a large
ratio of surface atoms to atoms in its bulk. As a variation of an old
joke, we can ask ourselves, "How many
gold atoms does it take to make gold?" If by gold we mean the
lustrous metal used to make
jewelry, it takes quite a few. In two previous articles,
Very White and Very Black, November 23, 2011, and
Black Gold, November 29, 2006, I wrote about "black gold," an assemblage of small gold particles looking quite unlike gold.[2-3] Likewise,
black versions of other metals can be prepared.[4]
Two teams of
scientists have recently published investigations of very small gold atomic clusters near the material's non-metal to metal transition. The first group, from
Carnegie Mellon University (Pittsburgh, Pennsylvania), the
University of Toledo (Toledo, Ohio), and
Brookhaven National Laboratory (Upton, New York), created atomically precise gold nanoparticles containing 133–gold atoms. The clusters were non-metallic.[5-6]
These clusters were produced as a core of gold atoms surrounded by 52 surface-protecting,
sulfur-containing,
thiolate ligand molecules.[6] Clusters of this type even have their own
Wikipedia page The sulfur binds to gold atoms, and the
molecules self-assemble into the clusters.[5] The clusters have an
icosahedral gold core, and the surface atoms attach to the sulfur atoms of the ligand molecules.[6]
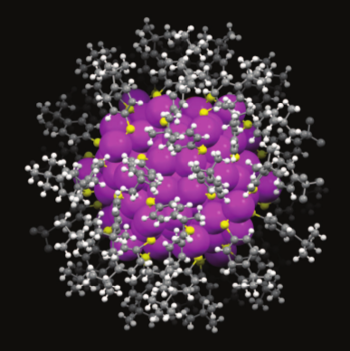
Thiolate ligand surrounded cluster of 133 gold atoms.
The gold atoms are magenta, the sulfur atoms are yellow, the hydrogen atoms are white, and the carboncarbon atoms are gray.
(Carnegie Mellon University image.)
X-ray crystallography was used to map the position of every atom on the cluster surface, and it was found that these were arranged in a
helical structure.[6] The ligand-surrounded gold nanoparticles were found to be nonmetallic by
optical and
electron dynamics measurements, so 133 gold atoms are not enough to make metallic gold.[5-6] This research was supported by the
Air Force Office of Scientific Research.[6]
The second research team, from the
Chemistry and
Physics Departments of the
University of Jyväskylä (Jyväskylä, Finland), looked at smaller, 120-atom, gold clusters.[7-8] Their clusters were surrounded by 44 molecules of
para-mercaptobenzoic acid (pMBA), and they used
ultrafast time-resolved mid-infrared spectroscopy and
density functional theory calculations to distinguish between metallic and non-metallic behavior.[7]
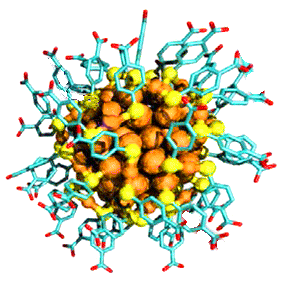
A cluster of 102 gold atoms surrounded by 44 molecules of pMBA.
(Modified University of Jyväskylä image.)
The 102-atom clusters were found to be non-metallic, while the team's previous research showed that a gold cluster of 144 atoms, surrounded by sixty molecules of SC
2H
4Ph (where Ph is a
phenyl group), is a metal.[7-8] Looking at the results of these
experiments, and the experiments on 133 atom clusters, shows that the transition between non-metallic and metallic behavior occurs between 133 and 144 gold atoms.[5-8]
Says
Mika Pettersson, a
professor at the University of Jyväskylä and the
principal investigator of the research team,
"Molecules behave drastically different from metals... The additional energy from light, absorbed by the metal-like clusters, transfers to the environment extremely rapidly, in about one hundred billionth of a second, while a molecule-like cluster is excited to a higher energy state and dissipates the energy into the environment with a rate that is at least 100 times slower. This is exactly what we saw: the 102-gold atom cluster is a giant molecule showing even a transient magnetic state while the 144-gold atom cluster is already a metal. We've thus managed to bracket an important size region where this fundamentally interesting change in the behavior takes place."[8]
In answer to our original question of how many gold atoms it takes to make gold, a good guess would be 140.
References:
- Mark E. Davis, and Robert J. Davis, "Heterogeneous Catalysis," Chapter 5 of Fundamentals of Chemical Reaction Engineering (2003) at the California Institute of Technology web site (PDF File).
- John Lehman, Evangelos Theocharous, George Eppeldauer and Chris Pannell, "Gold-black coatings for freestanding pyroelectric detectors," Measurement Science and Technology, vol. 14, no. 7 (July, 2003).
- W. Becker, R. Fettig and W. Ruppel, "Optical and electrical properties of black gold layers in the far infrared," Infrared Physics & Technology, vol. 40, no. 6 (December, 1999), pp. 431-445.
- Chih-Ming Wang, Ying-Chung Chen, Maw-Shung Lee and Kun-Jer Chen, "Microstructure and Absorption Property of Silver-Black Coatings," Jap. J. Appl. Phys. vol. 39, Part 1, no. 2A, (February 15, 2000) pp. 551-554.
- Chenjie Zeng, Yuxiang Chen, Kristin Kirschbaum, Kannatassen Appavoo, Matthew Y. Sfeir, and Rongchao Jin, "Structural patterns at all scales in a nonmetallic chiral Au133(SR)52 nanoparticle," Science Advances, vol. 1, no. 2 (March 20, 2015), Document No. e1500045, DOI: 10.1126/sciadv.1500045. This is an open access article with a PDF file available here.
- Jocelyn Duffy, "CMU Chemists Create Tiny Gold Nanoparticles That Reflect Nature's Patterns," Carnegie Mellon University Press Release, April 8, 2015.
- Satu Mustalahti, Pasi Myllyperkiö, Sami Malola, Tanja Lahtinen, Kirsi Salorinne, Jaakko Koivisto, Hannu Häkkinen, and Mika Pettersson, "Molecule-like Photodynamics of Au102(pMBA)44 Nanocluster," ACS Nano, vol. 9, no. 3 (February 22, 2015), pp 2328-2335, DOI: 10.1021/nn506711a.
- How many gold atoms make gold metal?, Academy of Finland Press Release. May 11, 2015.
Permanent Link to this article
Linked Keywords: Atom; surface; crystal; chemical; dangling bond; atomic orbital; chemical bond; bonded; electron; heterogeneous catalysis; catalytic; material; catalyst; chemical reaction; reacting; precursor chemical; platinum; rhodium; vacancy defect; adatom; Inkscape; nanocrystal; ratio; joke; gold; lustre; lustrous; metal; jewelry; black; scientist; Carnegie Mellon University (Pittsburgh, Pennsylvania); University of Toledo (Toledo, Ohio); Brookhaven National Laboratory (Upton, New York); sulfur; thiol; thiolate; ligand molecule; thiolate-protected gold cluster; icosahedron; icosahedral; hydrogen; carbon; X-ray crystallography; helix; helical; optics; optical; electronic correlation; electron dynamics; Air Force Office of Scientific Research; Chemistry; Physics; University of Jyväskylä (Jyväskylä, Finland; thiosalicylic acid; para-mercaptobenzoic acid; ultrashort pulse; ultrafast time-resolved; mid-infrared; spectroscopy; density functional theory; calculation; phenol; phenyl; experiment; Mika Pettersson; professor; principal investigator; energy; light; second; dissipation; dissipate; magnetic field.