Breath Analysis
December 7, 2015
There's a
joke that
bad breath is better than no
breath at all. With all of today's aids to
oral hygiene, there's no reason for most people to have bad breath, except after eating certain
foods, such as
garlic and
onions. Mouth odors can also be
diagnostic of certain
medical conditions.
Ketoacidosis, a condition caused by untreated
diabetes, can cause the breath to smell like
acetone.
Sinusitis will cause an odor on a person's breath, as will
tobacco smoking. In what I consider just
anecdotal evidence, a 1930s
marriage manual claimed that an odor of
semen could be detected on a woman's breath within an hour after
sexual intercourse.[1] Perhaps I should write a
proposal to fund a
scientific study on this.
All these are odors detectable by the human sense of smell (
olfaction), which is not that sensitive. Although
experimental evidence is lacking, and an
experiment would be difficult to perform, it's acknowledged that
dogs are much better at
scent recognition than
humans. Just as we have
amplifiers for our hearing, there are also
instruments that detect faint odors.

"Do you hear me, now?"
More than 3 million hearing aids are dispensed annually in the United States.
(Portion of a Wellcome Trust image,
A woman shouting into a man's ear-trumpet, via Wikimedia Commons.)
Over the years,
scientists have developed devices that act as
artificial noses. The simplest of these involves heated
tin oxide that changes its
electrical conductivity upon exposure to
reactive gases, such as
hydrogen and
carbon monoxide. While adequate for a certain range of applications, these hardly qualify as general purpose odor sensors.
As I wrote in a
previous article (Weighing Attograms, January 27, 2014),
resonant cantilevers can be used as sensitive detectors of mass changes. The cantilever, clamped at one end as shown in the figure, is well known to
mechanical engineers, since its properties are explained by some simple mathematics.
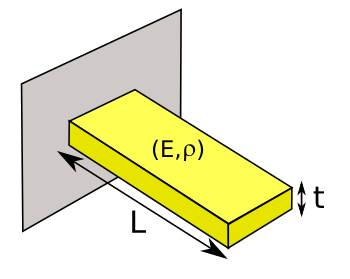
A cantilever beam.
Since the beam density is a determinant of the resonant frequency, molecules adsorbed on the surface of the beam can be detected.
(Illustration by the author, rendered using Inkscape.)
The resonant frequency f of such a cantilever is given as
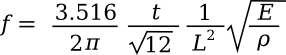
where
t is the beam thickness,
L is the beam length,
E is
Young's modulus, and ρ is the material density. Surprisingly, the width of the beam doesn't figure into the formula. If we coat the cantilever with a material that selectively adsorbs a certain type of
molecule from the air, we can sense that odor.
MEMS technology has enabled creation of arrays of a large number of such cantilevers, so our artificial nose can detect many odors at one time.
While arrays of such cantilevers can serve as sensors for a certain spectrum of odors, their
fabrication takes a lot of time and resources, and we still need to identify an appropriate group of
chemical coatings to adsorb the molecules of interest.
Optical spectroscopy allows creation of a more general type of odor sensor.
Chemists routinely use
infrared spectrometers to identify
chemical compounds, since chemical compounds have an unique
infrared signature arising from their different
bonds. In 1934, Richard Badger proposed a
rule that stronger bonds have higher
vibrational frequency.[2] The logic of this can be seen in the
analogy with the
frequency of a stretched
guitar string. Infrared spectra are extremely useful for chemical compound identification, so spectra for the important chemical compounds have been tabulated. An example can be seen in the figure, below.[3]
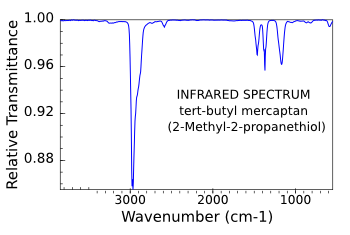
Infrared spectrum of tert-butyl mercaptan, a chemical added to natural gas to make leaks more detectable.
(Data from the NIST Chemistry WebBook.)[3)]
For really sensitive measurements, you can take spectroscopy one step farther with a technique called
cavity ring-down spectroscopy. The concept for this resembles my attempts as a
child to launch a
flashlight beam between
parallel mirrors. If you arrange two highly-
reflective parallel mirrors as shown in the figure, and then introduce a
light beam between them, the beam will reflect back and forth many times before
decaying to nothingness. For best effect, the
optical cavity must be
resonant at the
wavelength of the light source.
If there's a
vapor absorbing the light between these mirrors, then the light will decay faster. Since we're measuring a
time, and not an absolute
intensity, the technique rejects
noise to detect trace quantities of chemical compounds.
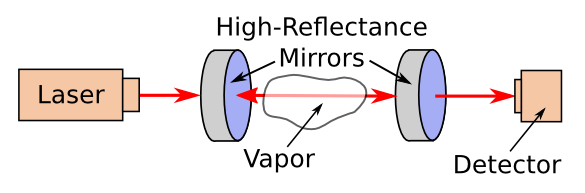
A laser-excited ring-down cavity. If we're able to tune the wavelength of the laser light, which is difficult, or use a broadband light source with filters at the detector, we can create a sensitive infrared spectrometer. Although the mirrors are highly reflective, they let a little light through to allow a pulse of light from the laser to enter, and light to exit to excite the detector. (Created by the author using Inkscape.)
It's easy to
calculate the ring-down time constant
τ by analysis of the
decay curve. The decay curve follows the
function
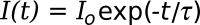
in which
I(t) is the intensity at a time
t and
Io is the initial intensity before the laser pulse is switched off. With sufficiently reflective mirrors, the light ray will bounce thousands of times, and the effective path length through the vapor can be a
kilometer. The absorbance of the vapor can be calculated from the ring-down time
τ.
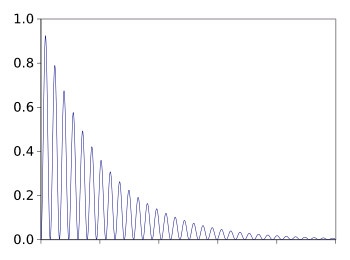
A ring-down curve for an optical cavity. The ring-down time is the point at which the intensity has decayed to 1/e (0.36788) of its initial value.
(Graphed by the author using Gnumeric.)
The starting point for
breath gas analysis was 1971, when
Linus Pauling and his
colleagues published a
paper showing that human breath contained more than 200
volatile organic compounds.[4] Since that time, a total of about 3,000 have been detected. Chemical compounds specific to certain diseases are present in just trace quantities. Today, there's even a
topical journal in this
research area, the
Journal of Breath Research, published since 2007.
Kris Newby, the communications manager for the
Stanford University (Stanford, CA) Center for Clinical and Translational Research and Education, has published an interesting article on recent research in using breath analysis for disease diagnostics. The article appears in the recent issue of
Stanford Medicine, a Stanford University publication.[5]
In 2013, three
graduate students at Stanford's
High Temperature Gas Dynamics Laboratory,
Christopher Strand,
Victor Miller and
Mitchell Spearrin, were
brainstorming possible projects outside their chosen
aerospace field. After first considering a
marijuana breathalyzer, they focused on the possibility of a disease breathalyzer. Their first target was
ammonia gas, the sign of a serious
metabolic disease called
hyperammonemia.[5]
Not surprisingly, they decided that
mid-infrared spectroscopy would be a good detection means, and they received a small grant to produce a
prototype device within a year. In their device, a person blows into a tube and their breath enters a
pressure-regulated cylinder in the path between a laser beam and a photodetector. Their prototype was constructed on a table top, and it was designed to detect not only ammonia, but also
carbon dioxide as an indicator of the length of a breathing cycle.[5]
As they found, ammonia is a difficult gas to detect, since it's a
polar molecule that's inclined to
stick to surfaces, and it's highly
soluble in
water. As a consequence of its solubility, ammonia dissolves in the
mouth; and, as a consequence of its polarity, it's adsorbed on the walls of plastic tubing. After much
development work, the research team shrunk the device to a portable unit, and they obtained
permission for human subject testing, and that testing went well.[5]
There are plans for a larger human trial on young children, and funding has been secured from the
NIH Small Business Technology Transfer program and the
Wallace H. Coulter Foundation. I wrote about Wallace H. Coulter in a
previous article (Flow Cytometry, October 11, 2011). Stanford University has filed a
provisional patent application on this work, and the team has formed a company,
Lumina Labs, to
commercialize the technology.[5]
References:
- Mary Roach, "10 things you didn't know about orgasm," TED conference, May, 2009.
- Richard Badger, "A Relation Between Internuclear Distances and Bond Force Constants," J Chem. Phys., vol 2, no. 3 (March 1, 1934), pp. 128ff., doi:10.1063/1.1749433.
- NIST Standard Reference Database Number 69, NIST Chemistry WebBook.
- Linus Pauling, Arthur B. Robinson, Roy Teranishit, and Paul Cary, "Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid
Partition Chromatography,"Proc. Nat. Acad. Sci., vol. 68, no. 10 (October, 1971), pp. 2374-2376. A PDF file is available here.
- Kris Newby, "Rocket men - Analyzing the breath of critically ill children at warp speed," Stanford Spectrum, Fall 2015.
Permanent Link to this article
Linked Keywords: Joke; halitosis; bad breath; breathing; breath; oral hygiene; food; garlic; onion; diagnosis; diagnostic; disease; medical condition; ketoacidosis; diabetes mellitus type 1; acetone; sinusitis; tobacco smoking; anecdotal evidence; marriage; book; manual; semen; sexual intercourse; research proposal; scientific method; scientific study; olfaction; experimental; experiment; dog; odor; scent; human; hearing aid">amplifier; scientific instrument; United States; Wellcome Trust; Wikimedia Commons; scientist; machine olfaction; artificial nose; tin dioxide; tin oxide; electrical conductivity; chemical reaction; reactive; gas; hydrogen; carbon monoxide; resonance; resonant; cantilevers; mass; mechanical engineering; mechanical engineer; mathematics; cantilever beam; density; resonant frequency; adsorption; adsorbed; Inkscape; Young's modulus; molecule; microelectromechanical systems; MEMS; technology; microfabrication; chemical compound; coating; optical spectroscopy; chemist; infrared spectroscopy; infrared spectrometer; infrared; chemical bond; Badger's rule; vibration; vibrational frequency; analogy; frequency; guitar string; tert-butyl mercaptan; natural gas; NIST Chemistry WebBook; cavity ring-down spectroscopy; child; flashlight; parallel; mirror; reflection; reflective; light beam; exponential decay; optical cavity; resonance; resonant; wavelength; vapor; absorption spectroscopy; absorb; time; intensity; noise; laser; cavity ring-down spectroscopy; ring-down cavity; filter; photodetecto; pulse; Inkscape; calculation; calculate; function; kilometer; ring-down curve; optical cavity; Gnumeric; breath gas analysis; Linus Pauling; collaboration; colleague; scientific literature; paper; volatility; volatile; organic compound; topical journal; research; Journal of Breath Research; Kris Newby; Stanford University (Stanford, CA); Center for Clinical and Translational Research and Education; Stanford Medicine; postgraduate education; graduate student; High Temperature Gas Dynamics Laboratory; Christopher Strand; Victor Miller; Mitchell Spearrin; brainstorming; aerospace; cannabis; marijuana; breathalyzer; ammonia gas; metabolic disorder; metabolic disease; hyperammonemia; mid-infrared; prototype; pressure regulator; pressure-regulated; cylinder; carbon dioxide; chemical polarity; polar molecule; adhesion; stick; solubility; soluble; water; mouth; research and development; clinical trial protocol; permission for human subject testing; National Institutes of Health; NIH; Small Business Technology Transfer program; Wallace H. Coulter Foundation; provisional patent application; Lumina Labs; commercialization; commercialize.