Rust
December 19, 2014
When I was a
child in the
1960s, there were clear
gender roles, and no one was ranting about
gender inequality. It was rare that a
woman would work, so they were mostly happy
mothers and
homemakers. The men provided the
household income, and their time away from home allowed their
bonding with other males in the traditional
tribal ways.
At that time, women in the
Soviet Union needed to work, so they would leave their children at state-run
daycare centers. Everyone in the
US thought that this was cruel for both the mothers and children, and now we've defaulted to the same thing.
In those days, there was even a definite gender difference in the idea of rust.
One type of rust is a
fungus that attacks
flowering plants, such as
geraniums,
carnations,
asters,
pansies and
sunflowers.That was the rust that was on my mother's mind as she tended her
garden. When my
father heard the word, "rust," he thought instead of the
oxidation product of iron.
Nowadays, it's rare to hear anyone talk about garden flowers, let alone rust fungus. Everyone thinks of red
iron oxide (
hematite, Fe
2O
3) when they hear "rust." Iron has been a large part of our
culture since the
Industrial Revolution, and the rust that accompanies it is ubiquitous. When the US gave up on
manufacturing, following instead the lure of a false
finance culture, the remains of our manufacturing
infrastructure were aptly named the
Rust Belt.
As long as your iron doesn't have too much
surface area, as it would have in a fine
powder or
nanoparticle form, rusting happens at a very slow
rate. The rust
reaction,
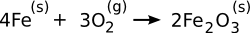
is spontaneous at all
temperatures above
room temperature, as can be seen from the
negative Gibbs Free Energy of its formation (see graph). This reaction is a good example that reactions found to be spontaneous by a free energy
calculation do not necessarily occur at a rapid rate. In rusting, a slow rate of reaction is good, but when you're producing some
chemical compound, a slow reaction rate might be a problem.
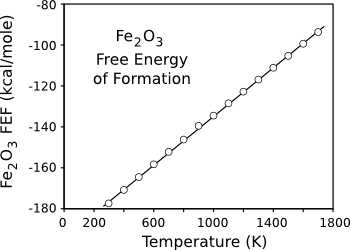
Negative free energy of reaction indicates a spontaneous reaction. However, "spontaneous" is not synonymous with "fast."
(Data plotted using Gnumeric.)
Red hematite isn't the only iron oxide.
Magnetite (Fe
3O
4), black iron oxide, is a common
mineral. It's a
magnetic material, and it's sometimes found in a naturally
magnetized state as
lodestone. Lodestone enabled early forms of the
compass. Hematite forms from magnetite by the following
oxidation reaction,
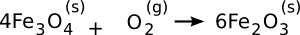
Since the Industrial Revolution started about 250 years ago, and human discovery of iron was about seven
millennia before that, you would think that we would know everything about the oxides of iron. Well, that doesn't seem to be the case, as
scientists from the
Vienna University of Technology (Vienna, Austria) and the
University of Erlangen-Nürnberg (Erlangen, Germany) have found that the arrangement of
atoms on the surface of magnetite is not what everyone had thought it to be.[2-3]
A ball-and-stick model.
Ball-and-stick modeling was made famous by Watson and Crick, but it's used by inorganic chemists and materials scientists as well.
(TU Wien image.)
The atomic-scale structure at surfaces is important, since the surface atoms are the first to participate in any reaction.[2] The surface state of magnetite is important, since it's used as a
catalyst, in
electronic devices and in
medical applications.[3] One unusual property of the magnetite surface is that atoms of
gold or
palladium,
deposited on its surface, stick where they attach and do not migrate to form nanoparticles. This is the reason why magnetite is an efficient catalyst, but the reason for this surface behavior was unknown.[3]
Such a
phenomenon would happen when there are
oxygen vacancies at the surface. What was unexpected was that the surface properties of magnetite arise from missing iron atoms, instead.[3] Says
Ulrike Diebold, a
professor at the Vienna University of Technology (also known as TU Wien) and the head of its metal-oxide-research group
"Because materials interact with their environment through the surface, it's really important to understand the structure of the surface and why it forms... It turns out that the surface of Fe3O4 is not Fe3O4 at all, but rather Fe11O16."[3]
The magnetite structure isn't an
array of iron atoms with inserted oxygen; rather, its an array of oxygen atoms with inserted iron. There are missing iron atoms in the layer just below the surface.[3] The positions above these missing iron atoms are where other metal atoms
attach. Not only that, but these positions for attachment are regularly-spaced, and the distance between such sites is large enough to prevent the attached surface atoms from
attracting each other.[3]
Quantitative
low-energy electron diffraction,
scanning tunneling microscopy, and
density functional theory calculations verified the structure.[2] The implication is that this phenomenon is not restricted to just magnetite, but it might occur in other oxides, such as the oxides of
cobalt,
manganese and
nickel.[2] The Vienna University of Technology has a web site, "Solids4fun," that promotes interdisciplinary
collaboration of materials and surface
science.[4]
Particle physicists aren't the only ones who usew large, expensive machines in their experiments. Here's the magnetite research team around some impressive vacuum equipment.
(TU Wien image.)[3)]
References:
- Free energy data from L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- R. Bliem, E. McDermott, P. Ferstl, M. Setvin, O. Gamba, J. Pavelec, M. A. Schneider, M. Schmid, U. Diebold, P. Blaha, L. Hammer, and G. S. Parkinson, "Subsurface cation vacancy stabilization of the magnetite (001) surface," Science, vol. 346 no. 6214 (December 5, 2014), pp. 1215-1218, DOI: 10.1126/science.1260556.
- Florian Aigner, "The finer details of rust," Vienna University of Technology Press Release, December 4, 2014.
- Solids4fun Web Site at the Vienna University of Technology.
Permanent Link to this article
Linked Keywords: Child; 1960s; gender role; gender inequality; woman; mother; homemaking; homemaker; household; income; male bonding; tribe; tribal; Soviet Union; day care; United States; rust fungus; flowering plant; geranium; dianthus caryophyllus; carnation; aster; pansy; helianthus; sunflower; garden; father; oxidation product of iron; iron; oxide; hematite; culture; Industrial Revolution; manufacturing; finance; infrastructure; Rust Belt; surface area; powder; nanoparticle; rate; chemical reaction; temperature; room temperature; negative number; Gibbs Free Energy; calculation; chemical compound; Gnumeric; Magnetite; mineral; magnet; magnetic material; magnetization; magnetize; lodestone; compass; oxidation; millennium; millennia; scientist; Vienna University of Technology (Vienna, Austria); University of Erlangen-Nürnberg (Erlangen, Germany); atom; ball-and-stick model; James D. Watson; Francis Crick; inorganic chemistry; inorganic chemist; materials science; materials scientist; TU Wien; catalysis; catalyst; electronics; electronic device; medicine; medical; gold; palladium; physical vapor deposition; phenomenon; oxygen; vacancy defect; vacancies; Ulrike Diebold; professor; crystal structure; array; chemical bond; van der Waals force; low-energy electron diffraction; scanning tunneling microscope; scanning tunneling microscopy; density functional theory; cobalt; manganese; nickel; collaboration; science; particle physicist; experiment; vacuum chamber; vacuum equipment.