Nanodiamond Thermal Conducting Fluid
April 23, 2014
The phrase, "
the whole is greater than the sum of its parts," is often attributed to
Aristotle (384 BC-322 BC), but there's nothing exactly like that in his works. Nonetheless, this statement is often true for
composite materials.
Epoxy wouldn't be quite as useful without the addition of
glass fibers to form
fiberglass.
The following is a formal definition of a composite found on
Wikipedia.
"Composite materials... are materials made from two or more constituent materials with significantly different physical or chemical properties, that when combined, produce a material with characteristics different from the individual components."
Although we usually think of composites only as
solids, the definition allows
fluids, also. We have fluid-fluid mixtures, in which one of these fluids is usually called an additive. One example of these is
motor oil, for which there are
numerous additives. There are also liquid-in-solid composites, such as
oil-impregnated bronze, used as a material for
bearings; and solid-in-liquid composites, such as
ink and
paint sol colloids.
The easy production of
nanoparticles has now allowed the development of much more useful sol colloids, principally since these particles can be
suspended and
dispersed in fluids (see, for example, refs. 1-2).[1-2]
Scientists from
Rice University (Houston, Texas), the
Council of Scientific and Industrial Research−
Central Electrochemical Research Institute (CSIR-CECRI,
Karaikudi, India), the
Indian Institute of Science (Bangalore, India) and the
University of Texas Pan American (Edinburg, Texas) have just published the results of their
research on using
nanodiamonds to enhance the
thermal conductivity of
mineral oil.[3-4]
Diamond is an excellent thermal conductor, having a
room temperature thermal conductivity of
22 W/cm-K. This is five times greater than the thermal conductivity of
copper, but if you form diamond from the
isotope 12C, it has a
theoretical thermal conductivity at 80
kelvin of about 2000 W/cm-K.[5]
Mineral oil has a thermal conductivity of just 0.00162 W/cm-K. This sounds small, but it compares favorably to
ethylene glycol (0.00258 W/cm-K), one of the best thermally conducting liquids, although still a far second to
water at 0.0058 W/cm-K. The Rice team investigated suspensions of nanodiamond in mineral oil, which is used as a standard
transformer oil.

Suspension of nanodiamond in mineral oil.
The concentration of nanodiamond in mineral oil was tested up to 0.1 weight-percent.
(Image: Ajayan Group/Rice University.)[4)]
This work extended the team's previous work on addition of
exfoliated layers of
hexagonal boron nitride in mineral oil.[6] They were able to form stable nanosuspensions with high
shelf life and a higher
electrical resistivity than that of pure mineral oil. Just a small weight fraction of boron nitride nanoparticles (0.01 wt-%) enhanced the thermal conductivity by a factor of nearly two at higher
temperatures.[6]
Nanoparticles of size less than 100
nanometers won't limit
fluid flow if their
concentration is low enough. The nanodiamond particles of the Rice research team were about six nanometers in diameter, and this produced mineral oil fluids with a higher thermal conductivity than those containing
oxide,
nitride or
carbide ceramics,
metals,
semiconductors or
carbon nanotubes.[3-4] Just a tenth weight-percent of nanodiamond increased the thermal conductivity of mineral oil by 70 percent at 373
kelvin, about 210 degrees
Fahrenheit (see figure).[3-4]
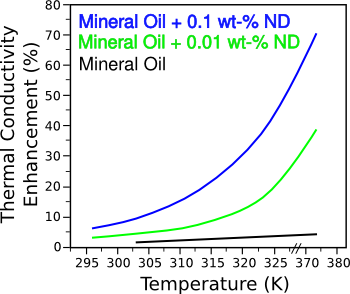
Enhancement of thermal conductivity by nanodiamond addition.
(Illustration by the author using Inkscape from Rice University data.)[3)]
An important consideration for most applications is the
fluid viscosity. The nanodiamond-filled mineral oil had a viscosity that had an
Arrhenius law behavior in which both the
activation energy and the
pre-exponential factor have an
exponential dependence on the fraction of nanodiamonds.[3] says
Taha-Tijerina, a coauthor of the paper and now a research scientist at
Viakable Technology and Research Center in
Monterrey, Mexico,
"The great properties of nanodiamond — lubricity, high thermal conductivity and electrical resistivity and stability, among others - are quite impressive... We found we could combine very small amounts with conventional fluids and get extraordinary thermal transport without significant problems in viscosity."[4]
It's thought that this large effect at such low concentration is achieved by a
percolation mechanism combined with
Brownian motion.[4]
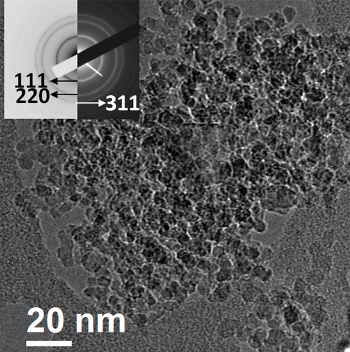
Electron microscope image of nanodiamond in mineral oil.
The inset is the electron diffraction pattern of the particles.
(Image: Ajayan Group/Rice University.)[4)]
![]()
References:
- Virginia A. Davis, A. Nicholas G. Parra-Vasquez, Micah J. Green, Pradeep K. Rai, Natnael Behabtu, Valentin Prieto, Richard D. Booker, Judith Schmidt, Ellina Kesselman, Wei Zhou, Hua Fan, W. Wade Adams, Robert H. Hauge, John E. Fischer, Yachin Cohen, Yeshayahu Talmon, Richard E. Smalley and Matteo Pasquali, "True solutions of single-walled carbon nanotubes for assembly into macroscopic materials," Nature Nanotechnology, vol. 4, no. 12 (December, 2009), pp. 830-834.
- Olga Matarredona, Heather Rhoads, Zhongrui Li, Jeffrey H. Harwell, Leandro Balzano, and Daniel E. Resasco,, "Dispersion of Single-Walled Carbon Nanotubes in Aqueous Solutions of the Anionic Surfactant NaDDBS," J. Phys. Chem., vol. B 2003, no. 107, pp. 13357-13367 (PDF File).
- Jose Jaime Taha-Tijerina, Tharangattu Narayanan Narayanan, Chandra Sekhar Tiwary, Karen Lozano, Mircea Chipara and Pulickel M. Ajayan, "Nanodiamond-Based Thermal Fluids," ACS Applied Materials & Interfaces, Article ASAP, March 21, 2014, DOI: 10.1021/am405575t.
- Mike Williams, "Diamonds are an oil's best friend," Rice University Press Release, March 31, 2014.
- Lanhua Wei, P. K. Kuo, R. L. Thomas, T. R. Anthony and W. F. Banholzer, "Thermal conductivity of isotopically modified single crystal diamond," Physical Review Letters, vol. 70, no. 24 (June 14, 1993), pp. 3764-3767.
- Jaime Taha-Tijerina, Tharangattu N. Narayanan, Guanhui Gao, Matthew Rohde, Dmitri A. Tsentalovich, Matteo Pasquali and Pulickel M. Ajayan, "Electrically Insulating Thermal Nano-Oils Using 2D Fillers," ACS Nano, vol. 6, no. 2 (February 28, 2012), pp 1214-1220.
- Ajayan Research Group at Rice University.
- Rice Materials Science and NanoEngineering Department.
Permanent Link to this article
Linked Keywords: Holism; the whole is greater than the sum of its parts; Aristotle (384 BC-322 BC); composite material; epoxy; glass fiber; fiberglass; Wikipedia; solid; fluid; motor oil; oil additive; additive; oilite; oil-impregnated bronze; bearing; ink; paint; sol; colloid; nanoparticle; suspension; dispersion; scientist; Rice University (Houston, Texas); Council of Scientific and Industrial Research; Central Electrochemical Research Institute; Karaikudi, India; Indian Institute of Science (Bangalore, India); University of Texas Pan American (Edinburg, Texas); research; nanodiamond; thermal conductivity; mineral oil; diamond; room temperature; material properties of diamond - Thermal conductivity; 22 W/cm-K; copper; isotopes of carbon; isotope; theory; theoretical; kelvin; ethylene glycol; water; transformer oil; Ajayan Group/Rice University; exfoliated layers; hexagonal crystal system; hexagonal; boron nitride; shelf life; electrical resistivity; temperature; nanometer; fluid flow; concentration; oxide; nitride; carbide; ceramic; metal; semiconductor; carbon nanotube; kelvin; Fahrenheit; Inkscape; fluid viscosity; Arrhenius law; activation energy; pre-exponential factor; exponential function; exponential dependence; Jaime Taha-Tijerina; Viakable Technology and Research Center; Monterrey, Mexico; percolation theory; percolation mechanism; Brownian motion; transmission electron microscopy; Electron microscope; electron diffraction.