Microengines
March 31, 2014
Batteries have become smaller and more powerful in recent years, but power-hungry
mobile device displays have become larger at the same time. I'm amazed that my
cellphone,
tablet computer and
netbook work so long on a single
charge; but, sometimes, it would be nice if the battery charge would last just a little longer.
The problem is the limited
energy density possible in an
electrochemical cell (a.k.a., battery). As the table shows, the energy density of today's battery technology is much less than that of other
energy sources.
A competent
engineer will strive to increase the
energy conversion efficiency of a
system to extract as much useful energy as possible; and, as the table shows, switching from one energy system to another can have a big affect. If you power your mobile electronic devices with the electrochemistry of
lithium ion battery, you get just 2.5
kJ/
gram. If it were possible to use butane combustion, the energy density jumps twenty-fold.
This is the approach taken by
Lilliputian Systems, Inc., which has developed a
USB recharger fueled by butane. This
MIT spin-off is
manufacturing its "Nectar" device, somewhat larger than a cellphone, that incorporates a
solid oxide fuel cell for conversion of butane and the
oxygen in
air to
electrical power.[1] Some major
engineering and
regulatory problems needed to be addressed in the design of this device.
Butane will
combust only at
temperatures above 400
°C (750
°F), and a fuel cell needs to operate at about 500°C (900°F) to use butane.[1] As can be imagined,
thermally insulating the fuel cell from its surroundings is important for both
safety and for extracting as much useful energy as possible.
Silicon is not a useful
electronic material above about 125°C, but it's an inexpensive and useful
structural material beyond that, since the
melting point of silicon is 1,414°C. The Nectar fuel cell is
micromachined from silicon, and the silicon is
nitrided to Si
3N
4 to reduce the
thermal conductivity.[1] Silicon has a thermal conductivity of about 150 W/m-K, while
silicon nitride's thermal conductivity can be as low as 10 W/m-K.[2]
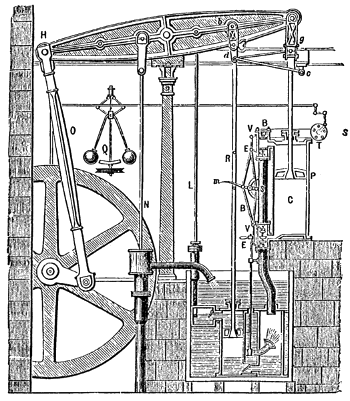
Old school energy conversion - The Boulton Watt steam engine, circa 1784.
This engine incorporated a water-cooled condenser, which increased the efficiency of steam engines of the time from 1% to 3%.
(Illustration from Robert H. Thurston, "A History of the Growth of the Steam Engine," D. Appleton and Company (New York, 1878), p. 119, via Wikimedia Commons.)
The fuel cell approach is useful if you want
electricity, but there are applications for which a small,
mechanical internal combustion engine would be useful. In 2011,
Yiguang Ju of
Princeton University (Princeton, NJ) and
Kaoru Maruta of
Tohoku University (Sendai, Japan) published a
review article, "Microscale combustion: Technology development and fundamental research," in the journal,
Progress in Energy and Combustion Science.[3] This 47-page paper is a useful resource on this topic that now includes engines for micro-
unmanned aerial vehicles.
One difficulty is scaling combustion motors to very small dimension is that it's difficult to create a combustion
reaction in a small
volume. The reaction
products quench the reaction and
heat flows too quickly to the environment. A team of
scientists from the
University of Twente (Enschede, the Netherlands), the
Russian Academy of Sciences, and the
University of Freiburg (Freiburg, Germany) have overcome this limitation, and they've demonstrated a combustion
actuator with the dimension 100 x 100 x 5
micrometers. It's based on the internal combustion of hydrogen and oxygen created by an
in situ electrolysis of water.[4-6]
The actuator is formed as a 530
nanometer thick membrane of silicon-rich nitride capping a small chamber through which water is allowed to flow (see photograph).[4-5]
Electrical current flow through the interdigital
electrodes electrolytically dissociates the water into oxygen and hydrogen, forming
nanobubbles. The nanobubbles create a slight
pressure of a few
atmospheres that moves the membrane.[4-5]
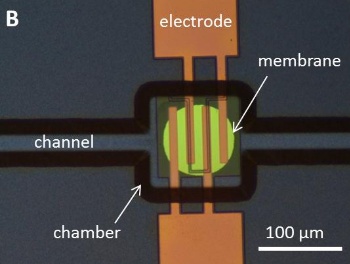
The electrolytic combustion microactuator.
(Fig. 1B of ref. 5, via arXiv.)
The interesting aspect of the engine cycle is that turning off the electrical current causes the bubble to rapidly collapse, an effect attributed to a recombination of the hydrogen and oxygen. Combustion is presumed, since the time of collapse is too short for the
gases to be dissolving back into the water.[4-5] Optimal operation for the device occurred at a 50
kHz alternating current, which might be related to the
resonance of the membrane.
References:
- Brian Dodson, "Miniaturized butane fuel cell system enables new USB battery charger," Gizmag, July 15, 2013.
- Xiang Zhang and Costas P. Grigoropoulosa, "Thermal Conductivity And Diffusivity Of Free-Standing Silicon Nitride Thin Films," Rev. Sci. Instrum., vol. 66, no. 2 (February 1995) pp. 1115-1120. A PDF file of this paper is available here.
- Yiguang Ju, Kaoru Maruta, "Microscale combustion: Technology development and fundamental research," Progress in Energy and Combustion Science, vol. 37, no. 6 (December 2011), pp. 669-715. A PDF file of this paper is available here.
- Vitaly B. Svetovoy, Remco G. P. Sanders, Kechun Ma and Miko C. Elwenspoek, "New type of microengine using internal combustion of hydrogen and oxygen," Scientific Reports, vol. 4, Article no. 4296 (doi:10.1038/srep04296, March 6, 2014.
- Vitaly B. Svetovoy, Remco G. P. Sanders, Kechun Ma and Miko C. Elwenspoek, "New type of microengine using internal combustion of hydrogen and oxygen," arXiv Preprint Server, February 27, 2014.
- The Powerful Promise of a Puzzling New Microscopic Combustion Engine, Technology Review, March 12, 2014.
Permanent Link to this article
Linked Keywords: Battery; batteries; mobile device; display device; mobile phone; cellphone; tablet computer; netbook; recharger; energy density; electrochemical cell; energy; lead acid battery; NiMH battery; lithium battery; ethanol; gasoline; butane; propane; methane; hydrogen; engineering; engineer; energy conversion efficiency; system; lithium ion battery; joule; kJ; gram; Lilliputian Systems, Inc.; Universal Serial Bus; USB; rechargeable battery; Massachusetts Institute of Technology; MIT; spin-off; manufacturing; solid oxide fuel cell; oxygen; atmosphere of Earth; air; electrical power; regulatory agency; combustion; temperature; Celsius; Fahrenheit; thermal insulation; thermally insulating; fire safety; silicon; semiconductor; electronic material; structural material; melting point; microelectromechanical system; micromachined; silicon nitride; thermal conductivity; thermal conductivity; Boulton Watt steam engine; condenser; Wikimedia Commons; electricity; mechanical; internal combustion engine; Yiguang Ju; Princeton University (Princeton, NJ); Kaoru Maruta; Tohoku University (Sendai, Japan); Progress in Energy and Combustion Science; unmanned aerial vehicle; chemical reaction; volume; product; heat transfer; heat flow; scientist; University of Twente (Enschede, the Netherlands); Russian Academy of Sciences; University of Freiburg (Freiburg, Germany); actuator; micrometer; electrolysis of water; nanometer; electrical current; electrode; electrolytically dissociate water; nanobubble; pressure; atmosphere; arXiv; gas; hertz; kHz; alternating current; resonance.