A Graphene-Carbon Nanotube Composite
December 3, 2014
When I worked in
corporate research, one
business principle that came into play often when considering
mergers and acquisitions was the idea of
synergy. Synergy, from the
Greek word, for "working together" (synergos, συνεργος), was the concept that "the whole is greater than the sum of its parts;" that is, the combination of business entities would lead to
efficiency and the possibility of combined
product offerings.
Before you scoff at an
operation that makes 1 + 1 = 3, you should check out the
Banach–Tarski paradox. This
mathematical proof,
published in 1924 by the eminent pair of
Polish mathematicians,
Stefan Banach (1892-1945) and
Alfred Tarski (1901-1983), showed that it's possible to break a
sphere into a
finite number of pieces, and then reassemble these pieces to make two spheres the same size as the original.[1]
(Artist's impression of the Banach–Tarski Paradox by Benjamin D. Esham, via Wikimedia Commons.)
Joining dissimilar
materials into
composites is one way that
materials scientists make a whole that's better than the sum of its parts. A common example of this is
fiberglass, which is a composite of the inexpensive materials,
glass fiber and
epoxy. Glass, itself, is a
brittle and
dense material that's not much suited for the production of
structural components, but
drawn glass fiber in an epoxy
matrix becomes a lightweight,
moldable material of reasonable
strength.
Glass fiber composites are being replaced in high end applications by
carbon-fiber-reinforced polymer composites.
Carbon is the wonder material of the past few
decades, with its various
allotropic forms, such as
nanotubes,
bucky-balls and
graphene, showing unique and useful properties. This carbon revival comes just after the similarly impressive feat of
commercial production of
diamond-like carbon layers.
Materials scientists from
Rice University (Houston, Texas), the
University of Akron (Akron, Ohio), and
Tsinghua University (Beijing, China) have made a composite of the nanotube and graphene carbon allotropes to form a better
cathode for
dye-sensitized solar cells.[2-3] As shown in the figure, this cathode is formed from vertically-aligned nanotubes that are
bonded to graphene sheets.[3] I wrote about dye-sensitized solar cells, also called
Graetzel cells, in a
previous article (Dye-Sensitized Solar Cells, June 23, 2010).
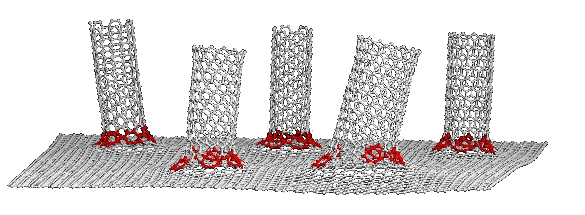
Carbon nanotubes, bonded to a graphene substrate. (Image: Tour Group/Rice University.)[3)]
Dye-sensitized solar cells are not as
efficient as
other types of solar cells, but they can be constructed from
environmentally benign materials, such as a
dye extracted from
raspberries, and
titanium dioxide; and, they don't need any exotic processing equipment, such as
vacuum chambers and
cleanrooms. The purpose of the dye is to absorb light at
solar wavelengths and transfer
electrons so
electrical current can flow in the cell. The composite
electrode replaces the brittle, and expensive,
platinum electrode on
tin oxide coated
glass usually used in these cells since it doesn't
degrade in the
electrolyte.[2-3]
Research always builds on what was discovered previously, and this electrode is no exception. In 2009, Rice University
chemist,
Robert Hauge, discovered a method of growing vertically-aligned carbon nanotubes using
catalytic particles that are raised by the nanotubes during growth to promote further growth. Then, in 2012, the method of doing the same on graphene was perfected to produce nanotubes bonded to the graphene substrate,[3] and graphene was subsequently produced on
metal grid as a
transparent electrode.[4]
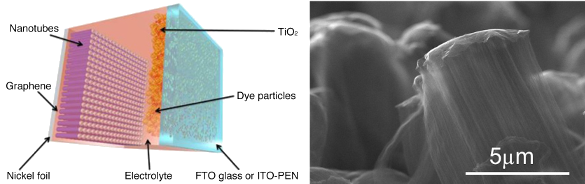
Left, construction of a Graetzel cell using the graphene-carbon nanotube electrode. Right, scanning electron microscope image of a group of vertically-aligned carbon nanotubes. (Left image and right image, N3L Research Group/Rice University.)[3)]
This composite electrode has a large
surface area, which is estimated as more than 2,000
square meters per
gram.[3] Since the carbon atoms of the nanotubes are bonded to the carbon atoms of the graphene substrate, the entire area is highly
conductive.[3] The
charge-transfer resistance of the cathode is twenty times smaller than that of platinum-based cathodes.[2-3] Solar cells fabricated at Rice had thickness up to 350
micrometers, which is the thickness of two sheets of
paper, so they could be flexed easily and repeatedly.[3]
Cathodes with the longest nanotubes performed the best, and these allowed a current of nearly 18
milliamps per
square centimeter. The platinum electrodes that they replace have a
current density of 14 mA/cm
2. The dye-sensitized solar cells produced with the new electrode material had
efficiencies of up to 8.2%, as compared with the 6.8% for the platinum-electrode cells.[3]
As is so much research in
Texas, this research was funded by the
Welch Foundation. Other support came from the
Air Force Office of Scientific Research, the
Department of Energy,
Lockheed Martin,
Sandia National Laboratory, and the
Office of Naval Research.[3]
References:
- Stefan Banach and Alfred Tarski, "Sur la décomposition des ensembles de points en parties respectivement congruentes". Fundamenta Mathematicae, vol. 6 (1924 ), pp. 244-277. (1.7 MB PDF File).
- Pei Dong, Yu Zhu, Jing Zhang, Feng Hao, Jingjie Wu, Sidong Lei, Hong Lin, Robert H. Hauge, James M. Tour, and Jun Lou, "Vertically Aligned Carbon Nanotubes/Graphene Hybrid Electrode as a TCO- and Pt-Free Flexible Cathode for Application in Solar Cells," Journal of Materials Chemistry A, vol. 2, no. 48 (Oct 31, 2014), pp. 20902-20907, DOI: 10.1039/C4TA05264A.
- Mike Williams, "Graphene/nanotube hybrid benefits flexible solar cells," Rice University Press Release, November 17, 2014.
- Pei Dong, Yu Zhu, Jing Zhang, Cheng Peng, Zheng Yan, Lei Li, Zhiwei Peng, Gedeng Ruan, Wanyao Xiao, Hong Lin, James M. Tour, and Jun Lou “Graphene on Metal Grids as the Transparent Conductive Material for Dye Sensitized Solar Cell,” J. Phys. Chem. C, vol. 118, no. 45(October 8, 2014), pp 25863-25868, DOI: 10.1021/jp505735j.
Permanent Link to this article
Linked Keywords: Corporation; corporate; research; business; mergers and acquisitions; synergy; Greek language; efficiency; product; operation; Banach–Tarski paradox; mathematical proof; scientific literature; publish; Polish; mathematician; Stefan Banach (1892-1945); Alfred Tarski (1901-1983); sphere; finite number; Benjamin D. Esham; Wikimedia Commons; material; composite; materials scientist; fiberglass; glass fiber; epoxy; brittleness; brittle; density; dense; structural material; interchangeable part; component; drawn glass fiber; matrix; molding; moldable; strength; carbon-fiber-reinforced polymer; carbon; decade; allotropes of carbon; allotropic form; carbon nanotube; buckminsterfullerene; bucky-ball; graphene; commerce; commercial; diamond-like carbon layer; Rice University (Houston, Texas); University of Akron (Akron, Ohio); Tsinghua University (Beijing, China); cathode; dye-sensitized solar cell; chemical bond; Graetzel cell; Tour Group/Rice University; energy conversion efficiency; solar cell; environmentally friendly; environmentally benign; dye; extraction; extract; raspberry; titanium dioxide; vacuum chamber; cleanroom; sunlight; solar wavelengths; electron; electric current; electrical current; electrode; platinum; tin oxide; glass; chemical decomposition; degradation; electrolyte; chemist; Robert Hauge; catalysis; catalytic; metal; grid; transparent; scanning electron microscope; N3L Research Group/Rice University; surface area; square meter; gram; electrical conductor; conductive; charge-transfer; resistance; micrometer; paper; ampere; milliamps; square centimeter; current density; Texas; Welch Foundation; Air Force Office of Scientific Research; United States Department of Energy; Lockheed Martin; Sandia National Laboratory; Office of Naval Research.