Graphene Production
May 26, 2014
There are several ways to make
scrambled eggs. The method I prefer is when the eggs are
whipped in a
bowl before
cooking, the resulting cooked portion being a very uniform blend of
yoke and
white. Another method is when the cracked eggs are added to the hot
frying pan, where they are
mixed,
in situ, but not very well. Some people may prefer the later result, but I think that if you're going to mix something, you should do it well, or not at all.
Blending of
powders and other
granular materials is an important
industrial processes for everything from
foodstuffs to
pharmaceuticals. A common
apparatus for doing such blending is the
double-cone "V" blender, as shown in the simplified
diagram, below.
Slow
rotation of the "V" shaped container shifts the contents from the
apex of the "V" to the two
prongs. At each rotation, the contents are divided in half, so the process is a lot like
shuffling cards. A large number of rotations ensures that the contents are well blended, just as a large number of shuffles will
randomize a
deck of cards. Blending time can be as long as fifteen
minutes.
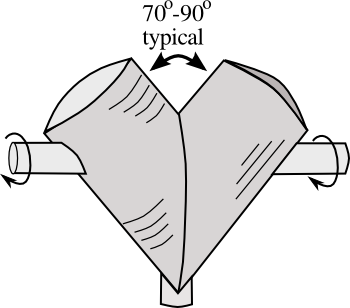
Simplified diagram of a "V" blender.
(Illustration by the author using Inkscape.)
This
process looks good on paper, but there might be problems when mixing some
materials. The card shuffling
analogy assumes that all the cards are identical, and no cards stick together. Materials having different sized and shaped particles might be a problem, as would
static electricity, generated by the granules'
rubbing together, holding particles together.
The slow
mechanical action of a "V" blender contrasts sharply with the rapidly rotating
blades in a
kitchen blender. Early
electric motor technology was fairly primitive, so the first blenders required a heavy motor, which was mounted in a base. A
jar with the rotating blades at its bottom was inverted onto the motor. Modern blenders are smaller, handheld devices that can be immersed into most containers.
Kitchen blenders can be used to chop
solids, such as
bread crumbs and
coffee beans, but they're typically used for creating
purées or
liquid mixtures such as
pancake batter. Kitchen blenders, and their
industrial cousins, are often seen in
chemistry laboratories, since they are used there for similar non-food mixing tasks. A team of more than 25
scientists from
Ireland and the
UK has just
published its discovery that a laboratory blender can be used to
exfoliate graphene sheets from
graphite.[1-4]
The
research team of
chemists,
physicists and
materials scientists from
Trinity College Dublin (Dublin, Ireland),
Thomas Swan and Company Limited (Consett, UK),
STFC Daresbury Laboratories (Warrington, UK) and
Oxford University (Oxford, UK) subjected mixtures of graphite flake in liquid
solvents to the mixing action of a
Silverson Machines Ltd. LSM high
shear laboratory mixer in a demonstration of an industrially scalable process for production of large quantities of
defect-free graphene.[1-2]

Caution advised.
Jacob Lanphere, a Ph.D. candidate at the The University of California, Riverside, holding a solution of graphene oxide.
A UCR study found that graphene oxide nanoparticles are very mobile in waterways, and they would have a negative environmental impact if released.
(University of California, Riverside, photograph.)
The 250
watt motor of the Silverson Machines mixer rotates a 50 mm mixing head at 6000
rev/min under load.[2] As
Count Rumford proved in his
cannon boring experiments, mechanical action such as this generates
heat. For that reason, the mixing vessel is placed in a
water bath at 15
°C.[2]
The team identified the processing
parameters under their control. These included mixing
time, mixing
speed, mixing
volume,
rotor diameter, graphite
concentration, rotor-
stator gap, rotor-stator
position in the liquid volume, the presence/number/configuration of baffles, graphite pre-treatment, graphite type, and solvent type.[2]
Solvents were selected based on their
surface energy (or
solubility parameter) close to that of graphene itself. This results in a low
mixing energy, which leads to stabilization of the exfoliated graphene flakes against reaggregation.[2]
organic solvents used in this study were
N-methyl-2-pyrrolidone and
N-cyclohexyl-2-pyrrolidone. Also suitable are
aqueous surfactant solutions, such as
sodium cholate in water, and some
polymer solutions.[2]
The research team demonstrated that high-shear mixing of graphite in such solvents results in
dispersions of exfoliated graphene
nanosheets in liquid volumes as small as a few hundred of
milliliters up to hundreds of
liters.[1,3] Exfoliation was found to occur when the local shear rate exceeds 10
4 s−1. These flakes were verified as
unoxidized and free of
basal-plane defects by
X-ray photoelectron spectroscopy and
Raman spectroscopy.[1]
Funding for this project was provided jointly by Thomas Swan Ltd. and the
Science Foundation Ireland.[3] Thomas Swan Ltd. has commercialized this discovery with two products, Elicarb® Graphene Powder and Elicarb® Graphene Dispersion.[3] This process can be applied to exfoliate
boron nitride,
molybdenum disulfide (MoS
2) and other layered crystals.[1]
After you place graphene on a
substrate, the next step would be its modification to produced
electronic devices such as
transistors. That's the topic of research published in a recent issue of
Nature Materials by scientists from the
University of Arizona (Tucson, Arizona), the
Massachusetts Institute of Technology (Cambridge, Massachusetts),
Harvard University (Cambridge, Massachusetts), the
US Army Research Laboratory (Adelphi, Maryland), the
National Institute for Materials Science (Tsukuba, Japan), and the
Instituto de Ciencia de Materiales de Madrid (ICMM-CSIC, Madrid, Spain).[5-6]
Working with something as small as graphene requires a special tool, and that's the
scanning tunneling microscope (STM), which is also used to image graphene surfaces. Although the
bonding within graphene sheets is very strong, the inter-sheet bonding is weak, so it's possible to slide one layer on another.
carbon atoms in three stacked graphene layers can arrange themselves in two different configurations,
Bernal and
rhombohedral. In the Bernal stacking, the carbon atoms of the top layer are directly above the atoms in the lower layer. In rhombohedral stacking, they're aligned with the holes in the bottom layer.[6]
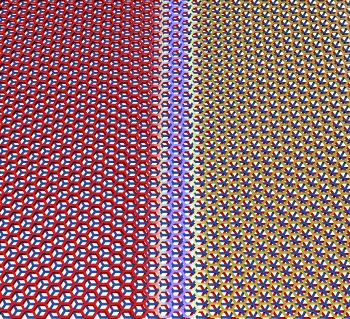
Trilayers of graphene sheets can stack in two different configurations, which can co-exist in a single flake with a transition region in between.
(Pablo San-Jose ICMM-CSIC image, via University of Arizona.)
(Click for larger image)
These exhibit very different
electronic properties, the Bernal stacking leading to a
semiconductor, while the rhombohedral forms an
insulator.[5] Both stacking configurations can coexist on a single graphene flake, and the region between them, called a
domain wall, accommodates the
strain with a modified
carbon–carbon bond distance.[5-6] The research team found that the applied
electric field of a scanning tunneling microscopy tip can move this domain wall.[6]
Study
coauthor,
Brian LeRoy, an
associate professor in the
University of Arizona Department of Physics, explains that "It is extremely rare for a material to change its
crystal structure just by applying an electric field... Making trilayer graphene is an exceptionally unique system that could be utilized to create novel devices."[6]
The research team found that the
free energy difference between the two stacking states scales with the
second power of the electric field, so that the rhombohedral stacking is favored at higher electric fields.[5] They speculate that use of a wide, knife-edged
electrode, rather than the single point, might allow movement of a domain over larger areas.[6]
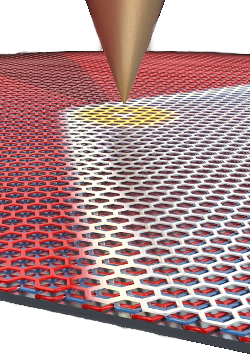
The metal tip of a scanning tunneling microscope can be used to move the domain border between different graphene configurations in the same flake.
Pablo San-Jose ICMM-CSIC image, via (University of Arizona.)
![]()
References:
- Keith R. Paton, Eswaraiah Varrla, Claudia Backes, Ronan J. Smith, Umar Khan, Arlene O'Neill, Conor Boland, Mustafa Lotya, Oana M. Istrate, Paul King, Tom Higgins, Sebastian Barwich, Peter May, Pawel Puczkarski, Iftikhar Ahmed, Matthias Moebius, Henrik Pettersson, Edmund Long, João Coelho, Sean E. O'Brien, Eva K. McGuire, Beatriz Mendoza Sanchez, Georg S. Duesberg, Niall McEvoy, Timothy J. Pennycook, et al., "Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids," Nature Materials, April 20, 2014, doi:10.1038/nmat3944.
- Supplementary Information for ref. 1.
- AMBER in world-first Graphene Innovation, Centre for Research on Adaptive Nanostructures and Nanodevices Press Release, April 22, 2014.
- Rachel Courtland, "Graphene You Can Whip Up In A Blender," IEEE Spectrum, April 21, 2014.
- Matthew Yankowitz, Joel I-Jan Wang, A. Glen Birdwell, Yu-An Chen, K. Watanabe, T. Taniguchi, Philippe Jacquod, Pablo San-Jose, Pablo Jarillo-Herrero andBrian J. LeRoy, "Electric field control of soliton motion and stacking in trilayer graphene," Nature Materials, advance online publication, April 28, 2014, doi:10.1038/nmat3965.
- Daniel Stolte, "Playing Pool with Carbon Atoms," University of Arizona Press Release, April 30, 2014.
Permanent Link to this article
Linked Keywords: Scrambled eggs; whisk; whipped; bowl; cooking; egg yolk; egg white; frying pan; mixing; blending; powder; granular material; industrial process; foodstuffs; pharmaceuticals; apparatus; industrial mixer">double-cone "V" blender; diagram; rotation; apex; prong; shuffling cards; randomization; randomize; playing card; deck of cards; minute; Inkscape; batch production; process; looks good on paper; material; analogy; static electricity; rubbing; mechanics; mechanical; blade; kitchen; blender; electric motor; electronics technology; jar; solid; bread crumb; coffee bean; purée; liquid; mixtures; pancake; batter; industry; industrial; chemistry; laboratory; scientist; Ireland; United Kingdom; UK; scientific literature; publish; exfoliation; exfoliate; graphene; graphite; research; chemist; physicist; materials scientist; Trinity College Dublin (Dublin, Ireland); Thomas Swan and Company Limited (Consett, UK); STFC Daresbury Laboratories (Warrington, UK); Oxford University (Oxford, UK); solvent; Silverson Machines Ltd.; shear stress; crystallographic defect; Jacob Lanphere; Doctor of Philosophy; Ph.D.; The University of California, Riverside; graphite oxide; graphene oxide; nanoparticle; waterway; environmental impact; watt; revolutions per minute; rev/min; Benjamin Thompson; Count Rumford; cannon; boring; experiment; heat; water bath; celsius; °C; parameter; time; speed; volume; rotor; diameter; concentration; stator; position; surface energy; solubility parameter; entropy of mixing; mixing energy; organic; N-methyl-2-pyrrolidone; N-cyclohexyl-2-pyrrolidone; aqueous; surfactant; cholic acid; sodium cholate; polymer; dispersion; nanosheet; milliliter; liter; second; oxide; unoxidized; basal-plane; X-ray photoelectron spectroscopy; Raman spectroscopy; Science Foundation Ireland; boron nitride; molybdenum disulfide; substrate; electronic device; transistor; Nature Materials; University of Arizona (Tucson, Arizona); Massachusetts Institute of Technology (Cambridge, Massachusetts); Harvard University (Cambridge, Massachusetts); United States Army Research Laboratory (Adelphi, Maryland); National Institute for Materials Science (Tsukuba, Japan); Instituto de Ciencia de Materiales de Madrid (ICMM-CSIC, Madrid, Spain); scanning tunneling microscope; chemical bond; bonding; carbon; atom; Bernal; rhombohedral; Pablo San-Jose ICMM-CSIC; electronic properties; semiconductor; insulator; domain wall; strain; carbon–carbon bond; electric field; coauthor; Brian LeRoy; associate professor; University of Arizona Department of Physics; crystal structure; gibbs free energy; quadratic equation; second power; electrode.