Glass
October 7, 2013
The emphasis in
materials science instruction for
my generation was
crystalline materials. Most of what we studied was
metallurgy, simply because the
mathematics to describe materials comprised of orderly arrays of
atoms is easy.
Glasses, which are
random networks of atoms, are harder to analyze mathematically. Today, modern
computer techniques, aided by improved
Xray and
electron diffraction instruments, have made glass an easier object of study.
William Zachariasen (1906-1979) was the first to propose the random network
model of glasses. Zachariasen was well qualified in the
physics of materials, since he had been a
post-doc of
Sir Lawrence Bragg and was invited to work at the
University of Chicago by
Arthur Holly Compton. Zachariasen, who also worked on the
Manhattan Project, remained at Chicago, serving twice as
chair of the physics department.
Zachariasen introduced his random network model in a 1932
paper, "The Atomic Arrangement in Glass."[1] In this model, atoms are
bonded to each other as you would expect from
valence bond theory, but their arrangement is not the periodic arrangement of
crystals.
Silica glass, for example, has
short-range order, since a
tetrahedral arrangement of
oxygen atoms around
silicon atoms is
energetically favored. These tetrahedra, however, will share oxygen atoms with other tetrahedral in a random arrangement.
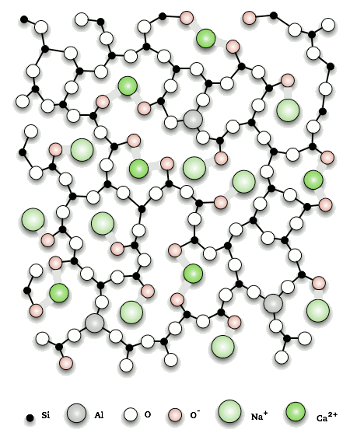
A possible arrangement of atoms in a calcium-sodium-alumino-silicate glass.
While crystals are usually particular on what atoms might be incorporated into their structure, glass compositions are generally less constrained.
(Via Wikimedia Commons.)
Before Zachariasen's time, glass was thought to be a collection of
nanocrystals, since X-ray diffraction experiments showed a single, broad diffraction peak and not the sharp peaks found for crystals. Based on the width of this peak, the nanocrystal size was calculated to be of the order of a few tens of
angstroms; that is, about ten
atom spacings. Zachariasen's model had the credible feature that it explained that the
strength of glass was of the same order as other solids, since the chemical bonding was the same.
Scientists always try to verify
theory by
experiment, and in 2012, an international collaboration by scientists at
Cornell University (Ithaca, New York), the
University of Ulm (Ulm, Germany), the
Max Planck Institute for Solid State Research (Stuttgart, Germany), the
University of Vienna (Vienna, Austria), the
University of Helsinki (Helsinki, Finland) and
Aalto University (Aalto, Finland) visualized the individual atoms in an extremely thin glass layer supported on
graphene using atomic resolution
transmission electron microscopy (see figure).[2] The atomic arrangement was as Zachariasen's random network model predicts. Now, this thin sheet of glass has won acclaim in non-scientific circles by being certified the world's thinnest sheet of glass by the
Guinness Book of World Records.
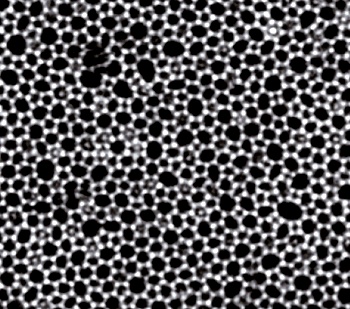
Micrograph of a thin silica glass sheet on graphene.
(Contrast-enhanced Cornell University image from ref. 2.)[2)]
As often happens in science, the process for formation of such glass sheets was an
accidental discovery by scientists at the University of Ulm.[2-5] During studies on graphene formation on
copper foil in a
quartz glass furnace, it was noticed that a layer had formed on the graphene. Investigations at Cornell University showed that the layer was silica glass formed by an
air leak that had caused the copper to react with the quartz glass, which, like silica, is a compound of silicon and oxygen.[4]
Atomic-resolution
electron spectroscopy at Cornell University showed that the glass was silica (SiO
2) formed from a bilayer of (SiO
4)
2– tetrahedra. There was no detectable
covalent bonding to the graphene.[2-3] The small thickness of the glass layer allowed its structure to be observed. The many atoms in thicker layers would produce a fuzzy image in which the structure couldn't be seen.[5]
Said
David A. Muller,
professor of
applied and engineering physics and director of the Kavli Institute at Cornell for Nanoscale Science,
"This is the work that, when I look back at my career, I will be most proud of... It's the first time that anyone has been able to see the arrangement of atoms in a glass."[5]
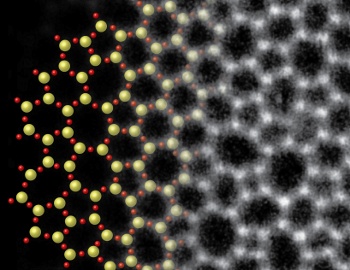
In this image, a micrograph of a two-atom thickness sheet of glass is blended with an artist's conception of its structure.
(Kavli Institute at Cornell for Nanoscale Science image.)[4)]
Muller says that this sheet of glass should be just slightly stronger than a
soap bubble.[5] The Guinness Book of World Records featured this thinest glass layer in its 21st Century Science spread.[4] The work at Cornell was funded by the
National Science Foundation.[4]
References:
- W. H. Zachariasen, "The Atomic Arrangement in Glass," J. Am. Chem. Soc., vol. 54, no. 10 (October 1932), pp 3841-3851.
- Pinshane Y. Huang, Simon Kurasch, Anchal Srivastava, Viera Skakalova, Jani Kotakoski, Arkady V. Krasheninnikov, Robert Hovden, Qingyun Mao, Jannik C. Meyer, Jurgen Smet, David A. Muller and Ute Kaiser, "Direct Imaging of a Two-Dimensional Silica Glass on Graphene," Nano Letters, vol. 12, no. 2 (January 23, 2012), pp 1081-1086.
- Ref. 2 as a PDF file on the Cornell University Web Site.
- Anne Ju, "Shattering records: Thinnest glass in Guinness book," Cornell Chronicle, September 12, 2013.
- Deborah Netburn, "Thinnest glass ever is just two atoms thick. Does it shatter?" LA Times, September 13, 2013.
Permanent Link to this article
Linked Keywords: Materials science; baby boomer; generation; crystal structure; crystalline materials; metallurgy; mathematics; atom; glass; random graph; random network; computer science; computer technique; X-ray crystallography; Xray; electron diffraction; scientific instrument; William Zachariasen (1906-1979); conceptual model; physics; postdoctoral research; post-doc; William Lawrence Bragg; Sir Lawrence Bragg; University of Chicago; Arthur Holly Compton; Manhattan Project; chairman; chair; scientific literature; paper; chemical bond; valence bond theory; crystal; silicon dioxide; Silica; short-range order; tetrahedral symmetry; oxygen; silicon; Helmholtz free energy; energetically favored; calcium-sodium-alumino-silicate glass; Wikimedia Commons; nanocrystal; angstrom; atomic spacing; strength; scientist; theory; experiment; Cornell University (Ithaca, New York); University of Ulm (Ulm, Germany); Max Planck Institute for Solid State Research (Stuttgart, Germany); University of Vienna (Vienna, Austria); University of Helsinki (Helsinki, Finland); Aalto University (Aalto, Finland); graphene; transmission electron microscopy; Guinness Book of World Records; serendipity; accidental discovery; copper; fused quartz; quartz glass; atmosphere of Earth; air; electron spectroscopy; covalent bond; David A. Muller; professor; applied and engineering physics; Kavli Institute at Cornell for Nanoscale Science; soap bubble; National Science Foundation.