Thin and Black
August 2, 2013
One interesting
experiment nearly every
child has done is to see what
color you get when you mix all your
paints together. The thrill of
science quickly subsides when he or she gets a
gray, murky mess. Paint works the way it does because its
pigments reflect light from specific colors and
absorb the others. The child's mixture has absorbers at nearly all color
wavelengths, but there's still an overall
broadband reflection. That's why the product is gray and not
black.
Black materials are useful for many applications. Black coatings will suppress stray light in sensitive
optical detectors, and black will give you maximum absorption of
solar energy. You must remember, however, that the black must extend to even those parts of the
solar spectrum you can't see, as shown in the figure. I wrote about the quest for super black materials in a
previous article (Very White and Very Black, November 23, 2011)
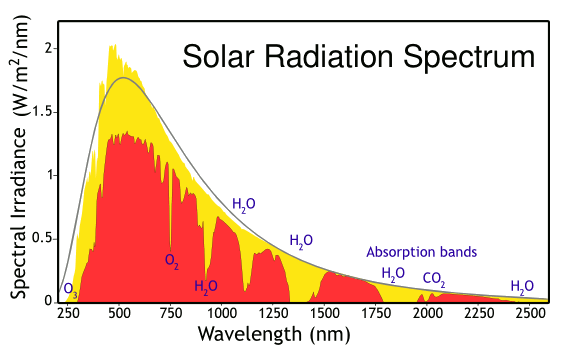
Solar energy incident at Earth's atmosphere and surface. The yellow curve is the radiation incident at the top of the atmosphere, while the red curve is the radiation at Earth's surface, diminished by the atmospheric absorbers shown. The radiation approximates a blackbody curve for 5250 kelvin. These data are from the American Society for Testing and Materials (ASTM) Terrestrial Reference Spectra, which is used as a test condition for solar panels in North America. (Modified image from Wikimedia Commons.)
The
optical properties of
nanomaterials are usually different from that of the bulk materials. One example of this is
black gold. When
gold and some other metals are evaporated under an
argon or
nitrogen pressure of about 750
millitorr, the gold
atoms cool and cluster before attaching to a
substrate to form a black layer.[1-3] Intense bursts of
femtosecond laser light on a gold layer will also produce black gold. The laser irradiation transforms the surface into a convoluted
nanoscale topography that's optically absorbent.[4]
When people think about black,
soot comes immediately to mind. Since soot is mostly
carbon, when
carbon nanotubes were discovered,
scientists had the idea that these could be a constituent of a
super-black material. In 2008, scientists from
Rensselaer Polytechnic Institute created a carbon nanotube material with a lower reflectance than any other material to date. Their super-black, a low-density array of vertically aligned carbon nanotubes, absorbed more than 99.955% of incident light at
visible wavelengths. Light incident on this forest of vertically aligned carbon nanotubes must make many multiple reflections to exit, losing energy at each reflection.[5-6]
Scientists from the
Japanese National Institute of Advanced Industrial Science and Technology and
Nagoya University prepared a similar array of vertically-aligned single-walled carbon nanotubes in 2009. Their material was a nearly perfect absorber over the extremely wide wavelength range from 0.2 - 200
μm.[7]
NASA prepared similar
multi-walled carbon nanotubes structures on a variety of substrates in 2011 (see photograph).[8]
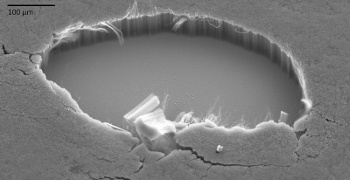
Iron-catalyzed multi-walled carbon nanotubes on silicon. A patch was removed to show the structure.
(Image: Stephanie Getty, NASA Goddard.)
The NASA material was prepared by using a thin
iron layer as a
catalyst for nanotube growth. This was achieved by heating them at 750
°C in the presence of carbon
feedstock gases.[8] The NASA material didn't qualify as super-black, since it absorbed just 99.5% of visible and
ultraviolet light and only 98% of far-
infrared light. Still, such numbers are useful because of the complementary
emissivity which would allow efficient
radiation of heat from
electronic components in
spacecraft.[8]
NASA continues to work on black materials with a goal of being able to coat the complex
three-dimensional components found on
spacecraft. For this reason, they've turned to
atomic layer deposition (ALD) of the
iron oxide catalyst that's placed on surfaces to induce the carbon nanotube growth. ALD is not a line-of-sight deposition technique, so it coats the bottom of deep notches as well as flat surfaces. The resultant black surfaces are very thin, just a few tens of
nanometers thick [9].
NASA relied on the expertise of the
Melbourne Centre for Nanofabrication for the atomic layer deposition. The
laboratory coated a number of NASA components, including an intricately-shaped optical
occulter intended to block
stellar light to allow observation of
planets around other
stars (see figure).[9]

Occulter mask in an ALD deposition fixture at the Melbourne Centre for Nanofabrication.
The mask is coated with an iron oxide catalyst prior to growth of a carbon nanotube layer.
(NASA image.)[9)]
Scientists at
Stanford University are also using atomic layer deposition to create thin, black absorbers, albeit near a particular wavelength of light, 600 nm.[10-11] Says
Stacey F. Bent, a
Professor of
Chemical Engineering at Stanford and a member of the research team, "Our results show that it is possible for an extremely thin layer of material to absorb almost 100 percent of incident light of a specific wavelength."[11]
The Stanford absorber is made from a planar sea of gold dots, about 14 nanometers tall and 17 nanometers wide (see figure).[11] These particles optically
resonate at 600 nm because of
plasmonic waves. The fabrication process used
block-copolymer lithography, which produced about 520 billion nanodots per
square inch in an
hexagonal array on
wafers. The gold dots are subsequently coated with
tin sulfide by atomic layer deposition to tune the resonance.[11]
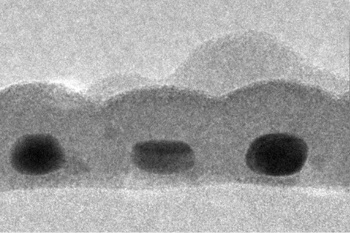
An electron micrograph showing a cross-section of the Stanford absorber layer. Three gold nanodots are seen, each about 14 nm by 7 nm in size, coated with tin sulfide.
(Image: Carl Hagglund, Stanford University.)[11]
These coatings, which were equivalent to a continuous gold coating just 1.6 nm thick, were still capable of absorbing 99% of the 600 nm light. The effective
absorption coefficient was 1.7 × 10
7 cm
-1.[10] Such a coating is three
orders of magnitude thinner than the absorbers used on commercial solar cells.[11] The next step is to demonstrate an improvement in solar cell
efficiency with this technology.[11]
Although gold was used in these layers, less-expensive
silver may perform better. Tin sulfide was used as a coating material, but
zinc oxide or
aluminum oxide may work as well. The research was supported by Stanford's
Center on Nanostructuring for Efficient Energy Conversion, an
Energy Frontier Research Center funded by the
U.S. Department of Energy.[11]
I often wear gloves like this.
Experimental samples of the black absorber.
(Image: Mark Shwartz, Stanford University.)[11]
![]()
References:
- John Lehman, Evangelos Theocharous, George Eppeldauer and Chris Pannell, "Gold-black coatings for freestanding pyroelectric detectors," Measurement Science and Technology, vol. 14, no. 7 (July, 2003), doi:10.1088/0957-0233/14/7/304.
- W. Becker, R. Fettig and W. Ruppel, "Optical and electrical properties of black gold layers in the far infrared," Infrared Physics & Technology, vol. 40, no. 6 (December, 1999), pp. 431-445.
- Chih-Ming Wang, Ying-Chung Chen, Maw-Shung Lee and Kun-Jer Chen, "Microstructure and Absorption Property of Silver-Black Coatings," Jap. J. Appl. Phys. vol. 39, Part 1, no. 2A, (February 15, 2000) pp. 551-554.
- Jonathan Sherwood, "Ultra-intense laser blast creates true 'black metal'," University of Rochester Press Release, November 21, 2006.
- Zu-Po Yang, Lijie Ci, James A. Bur, Shawn-Yu Lin and Pulickel M. Ajayan, "Experimental Observation of an Extremely Dark Material Made By a Low-Density Nanotube Array," Nano Lett., vol. 8, no. 2 (February, 2008), pp 446-451.
- Researchers Develop Darkest Man-Made Material, Inside Rensselaer, vol. 2, no. 2, January 31, 2008.
- Kohei Mizuno, Juntaro Ishii, Hideo Kishida, Yuhei Hayamizu, Satoshi Yasuda, Don N. Futaba, Motoo Yumura and Kenji Hata, "A black body absorber from vertically aligned single-walled carbon nanotubes," Proc. Natl. Acad. Sci., vol. 106, no. 15 (April 14, 2009), pp. 6044-6047.
- Lori Keesey and Ed Campion, "NASA Develops Super-Black Material That Absorbs Light Across Multiple Wavelength Bands," NASA Goddard Press Release No. 11-070, November 8, 2011.
- NASA Engineer Achieves Another Milestone in Emerging Nanotechnology, NASA Press Release, July 17, 2013.
- Carl Hägglund, Gabriel Zeltzer, Ricardo Ruiz, Isabell Thomann, Han-Bo-Ram Lee, Mark L. Brongersma and Stacey F. Bent, "Self-Assembly Based Plasmonic Arrays Tuned by Atomic Layer Deposition for Extreme Visible Light Absorption," Nano Lett., vol. 13, no. 7 (June 27, 2013), pp 3352-3357, DOI: 10.1021/nl401641v.
- Mark Shwartz, "Stanford scientists break record for thinnest light-absorber," Stanford Report (Stanford University), July 18, 2013.
Permanent Link to this article
Linked Keywords: Experiment; child; color; paint; science; grey; gray; pigment; reflection; reflect; light; absorption of electromagnetic radiation; wavelength; bandwidth; broadband; black; photodetector; optical detector; solar energy; solar spectrum; super black; Earth's atmosphere; lithosphere; Earth's surface; greenhouse gas; atmospheric absorber; blackbody curve; kelvin; ASTM International; American Society for Testing and Materials; solar panel; North America; Wikimedia Commons; optics; optical; nanomaterial; gold; argon; nitrogen; millitorr; atom; substrate; femtosecond laser; nanoscopic scale; nanoscale; topography; soot; carbon; carbon nanotube; scientist; super-black; Rensselaer Polytechnic Institute; visible spectrum; visible wavelength; Japanese National Institute of Advanced Industrial Science and Technology; Nagoya University; micrometer; μm; NASA; multi-walled carbon nanotube; Stephanie Getty; NASA Goddard; iron; catalysis; catalyst; celsius; °C; raw material; feedstock; gas; ultraviolet; infrared; emissivity; thermal radiation; electronic component; spacecraft; solid geometry; three-dimensional; component; atomic layer deposition; ALD; iron oxide; nanometer; Melbourne Centre for Nanofabrication; laboratory; occulting disk; occulter; stellar; planet; star; Stanford University; Stacey F. Bent; Professor; Chemical Engineering; resonance; resonate; surface plasmon; plasmonic wave; block-copolymer; photolithography; lithography; square inch; hexagonal array; wafer; >tin sulfide; electron micrograph; Carl Hagglund; attenuation coefficient; absorption coefficient; order of magnitude; energy conversion efficiency; silver; zinc oxide; aluminum oxide; Center on Nanostructuring for Efficient Energy Conversion; Energy Frontier Research Center; U.S. Department of Energy; nitrile rubber; glove.