Storing Heat
March 29, 2013
Many people where I live in
Northern New Jersey have the same wish about
climate; surprisingly, it doesn't concern
global warming. All of us would like to store the oppressive
heat of our
summer so it can be used in the
winter. Twenty-three
degrees of angle doesn't sound like much, but this
tilt of
Earth's axis with respect to its
orbital plane causes the
insolation to change over the course of the year, transforming degrees of angle to
degrees of temperature.
When I was a
child, my
father would take his
sons fishing at a large, secluded
pond in
Upstate New York. Few people knew about this pond; but, many decades prior, it was an industrial site for storing the cold of winter for use in the summer. There was an
ice house on the
shore, and the pond
ice was cut and stored under mounds of
sawdust insulation. Residential
iceboxes used the
phase change of ice to
water to extract
heat energy from
food, thereby keeping the food cool.
I reviewed the use of phase change to store energy in a
previous article (Solar Salt, July 30, 2010). The
Archimede solar energy plant near
Syracuse, Sicily, uses the
latent heat of fusion (also called the
enthalpy of fusion) of inexpensive
nitrates to store
solar energy. One particular mixture of nitrates, (NaNO
3)
7(KNO
3)
44(NaNO
3)
49 has a
liquidus temperature of 141°
C and a latent heat of fusion of 132.5
kJ/
kg. This mixture, called Hitec®, is sold by
Coastal Chemical Co., L.L.C., Houston, Texas.
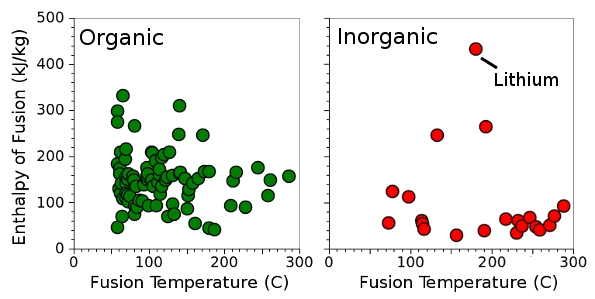
Enthalpy of fusion for 83 organic and 31 inorganic chemicals with melting points below 300°C. (Data collected by the author from various sources, graphed using Gnumeric.)
As can be seen from the above figure, a heat of fusion of 132.5 kJ/kg is high for an
inorganic chemical.
Lithium, at 432.2 kJ/kg, is a prominent exception, but molten lithium is
flammable, and it's not a good candidate for most applications. There are quite a few
organic chemicals with a high heat of fusion.
The latent heat of fusion of one organic compound has been put to good use in novel product,
Coffee Joulies™, for keeping
coffee hot. I mentioned Coffee Joulies™ in a
previous article (Coffee Thermodynamics, June 17, 2011). The chemical in these is not disclosed, but my bets are on
palmitic acid (hexadecanoic acid, CH
3(CH
2)
14CO
2H), which has a
melting point of 62.8° and an enthalpy of fusion of 209.5 kJ/kg.
Paraffin wax is not a specific chemical compound, but a mixture of
long-chain hydrocarbons, C
nH
2n+2. Paraffin wax melts at about 99°
F (37°C), and it has an enthalpy of fusion of about 200-220 kJ/kg, which puts it at the top of organic phase change materials. A team of
Chinese materials scientists has recently developed a convenient way to package paraffin wax for phase change energy storage.[1-2]

Slabs of paraffin, such as this, were common in kitchens of yesteryear. Molten food-grade paraffin was used to seal jars of home-made jellies and jams.
(Via Wikimedia Commons.)
This team of scientists from the School of Materials Science and Engineering,
Anhui University of Science and Technology (
Huainan, China), has developed a process to
micro-encapsulate paraffin in
silica (SiO
2) spheres. This
batch process involves coating paraffin droplets with
tetraethyl orthosilicate, commonly called TEOS, followed by
hydrolysis in an
ammonia solution to form silica.[1-2] The coating was aided by a
cetrimonium bromide (CTAB)
emulsifier, which is often used in the synthesis of gold nanoparticles.
The motivation for the research was the development of a phase change material without the mess; that is, having the liquid phase contained while still offering ample heat storage. Applications could include all-weather clothing, and
greenhouses. Most of the organic phase-change materials, including paraffin, are flammable, which is another disadvantage overcome by encapsulation.[2]
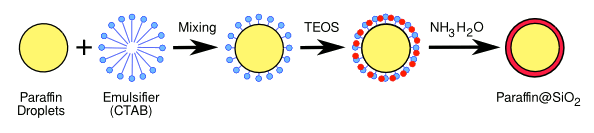
Process for encapsulation of microdroplets of paraffin in silica. Illustration by the author using Inkscape)
The process, as pictured above, produced quasi-
spherical particles with
diameters of 200–500
nm.[1] There was no
chemical reaction between the paraffin and the silica.[1]
Differential scanning calorimetry demonstrated a melting point of 56.5°C and an enthalpy of fusion of 45.5 kJ/kg.[1] This is considerably less than that of pure paraffin, since the paraffin comprised just 31.7% of the sphere volume, and there must be an allowance for the
packing fraction of the spheres, also.[1]
The paraffin-filled spheres cycled thirty times without change, and the spheres didn't leak after exposure to 70°C for twenty minutes.[2] The report on this work in
ACS Sustainable Chemistry & Engineering concludes that the reasonably high latent heat capacity and thermal stability of these spheres mark them as a possible material for some thermal energy storage applications.[1-2]. The research was funded by the
National Natural Science Foundation of China, the National Basic Research Program of China, and the Young and Middle-aged Backbone Teachers Fund of Anhui University of Science and Technology.[2]
References:
- Benxia Li, Tongxuan Liu, Luyang Hu, Yanfen Wang and Lina Gao, "Fabrication and Properties of Microencapsulated Paraffin@SiO2 Phase Change Composite for Thermal Energy Storage," ACS Sustainable Chem. Eng., vol. 1, no. 3 (2013), pp 374-380.
- Paraffin encapsulated in beach sand material as a new way to store heat from the sun, American Chemical Society Press Release, March 13, 2013.
Permanent Link to this article
Linked Keywords: North Jersey; Northern New Jersey; climate; global warming; heat; summer; winter; degrees of angle; axial tilt; Earth; axis; orbital plane; insolation; degrees of temperature; child; father; son; fishing; pond; Upstate New York; ice house; shore; ice; sawdust; thermal insulation; iceboxe; phase transition; phase change; water; heat energy; food; Archimede solar energy plant; Syracuse, Sicily; enthalpy of fusion; latent heat of fusion; enthalpy; nitrate; solar energy; liquidus; liquidus temperature; celsius; C; joule; kJ; gram; kg; Coastal Chemical Co., L.L.C.; organic compound; organic; inorganic compound; inorganic chemical; melting point; Gnumeric; lithium; flammability; flammable; Coffee Joulies™; coffee; palmitic acid; melting point; paraffin wax; long chain molecule; hydrocarbon; Fahrenheit; F; China; Chinese; materials scientist; Wikimedia Commons; Anhui University of Science and Technology; Huainan, China; micro-encapsulation; micro-encapsulate; silicon dioxide; silica; batch production; batch process; tetraethyl orthosilicate; hydrolysis; ammonium hydroxide; ammonia solution; cetrimonium bromide; surfactant; emulsifier; greenhouse; Inkscape; sphere; spherical; diameter; nanometer; nm; chemical reaction; differential scanning calorimetry; random close pack; packing fraction; ACS Sustainable Chemistry & Engineering; National Natural Science Foundation of China.