Nanomixing and Nanoparticle Thermodynamics
October 21, 2013
Nanoscale objects are interesting because they behave differently than larger objects. The
physics and
chemistry discovered for larger objects need to be modified for
nanoparticles. Some reasons for the different behavior of nanoparticles are the larger
ratio of
surface atoms to interior atoms, and their small size compared with a
wavelength of
light.
I mentioned some problems associated with mixing of
powders in a
previous article (Blending, March 11, 2008).
Liquid mixing is usually easier to accomplish, but there are still problems, as
oil and
water mixing demonstrates. The usual solution to getting
hydrophilic liquids (water) and
lipophilic (oil) liquids to mix is by adding a
surfactant. Surfactant
molecules have a hydrophilic head and a lipophilic tail, so they intermediate between the two different liquids.
A
research team from the
University of Washington (Seattle, Washington) and the
Pacific Northwest National Laboratory (Richland, Washington) published a paper earlier this year describing their
experiments on nanoscale mixing of liquids.[1-2] Their paper in the
Proceedings of the National Academy of Sciences demonstrates another example that nanoscale materials behave differently than their macroscale counterparts.[1] In this case, the effect likely arises from the fact that a greater number of molecules are at the
flow boundary than at the bulk.
The team used the device shown schematically in the figure. Mixing is done by passing liquids through posts with a
micrometer-sized gap. As the liquid flows through the device it
deforms quickly.[2] Their test liquids were mixtures of water,
cetyltrimethylammonium bromide, which is weakly
viscoelastic,
sodium salicylate, which is strongly viscoelastic and
shear-thinning, and a surfactant.[1]
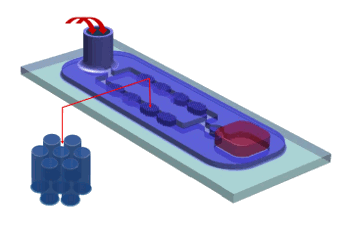
Schematic diagram of the nanofluidic mixer.
Liquids are injected at the back and forced through the posts for mixing.
(University of Washington image.)[2)]
When mixed this way, the liquids experienced
strain rates of about 10
3 s
−1 and strains of about 10
3. Under these conditions, the dissimilar liquids mix into entangled, branched, and multiconnected
micellar bundles. These are stable, because the mixing process makes it
energetically favorable to minimize the number of end caps and form cross-links.[1] The results can be seen in the
scanning electron micrograph in the following figure, which shows wormlike rods attaching and intertwining with each other.[2]

This electron micrograph shows the structure of a liquid with a gel-like consistency generated by the nanomixer in the previous figure.
(image: Environmental Molecular Sciences Laboratory of the University of Washington.)[2)]
Depending on the initial fluid mixture, the nanomixer will produce a mixed product that has more, or less,
viscosity. This research was funded by the
National Science Foundation.[2]
Nanoscale solids behave differently than their
macroscopic scale counterparts, a feature that's especially useful for
chemical processes such as
heterogeneous catalysis. Their
optical properties are often different from the bulk materials, as the different
coloration of nanoparticles of pure
metals has shown.[3] In a paper in
Physical Review Letters,
physicists from the
Vienna Center for Quantum Science and Technology and the
Vienna University of Technology (Vienna, Austria) have analyzed the
thermal properties of nanoparticles.[4-5]
The size of
optical absorbers and emitters is important to their
equilibrium state since it's smaller than the wavelengths of light which are absorbed or emitted. Large objects obey the
Planck radiation law, which describes the relationship between its temperature and
electromagnetic radiation; viz.,
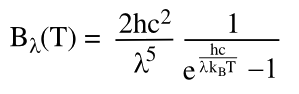
in which
B is the
spectral radiance,
T is the
absolute temperature,
kB is the
Boltzmann constant,
h is the
Planck constant, and
c is the
speed of light of the surrounding medium. Plotting this function gives you the familiar
blackbody curve.
Max Planck developed this law in 1900 as the first expression of
quantum mechanics, and it applies generally to a wide variety of objects from
stars to
charcoal briquettes, whether hot or at
room temperature. Planck himself realized that the law would not apply to very small objects, since an object cannot efficiently radiate thermal energy at wavelengths larger than its dimension.[5] An example from
electrical engineering would be the inefficient
transmission and
reception of
radio waves by a short
antenna.
The Vienna University of Technology team developed a generalized radiation formula from
first principles that applies at the nanoscale to arbitrarily-shaped objects.[4] They tested their formula using a
silica fiber with a diameter of 500
nanometer, which is smaller than a
thermal wavelength.[4] They were able to measure the fiber temperature very accurately by measuring its optical path length, and they were able to relate this to the optical energy.[5]
A picture, even an artist's conception, is worth a thousand words.
A thinned optical fiber as used in the study.
(Vienna University of Technology image.)
Arno Rauschenbeutel, an author of the paper, summarized its findings by saying,
"We could show that the fibers take much longer to reach their equilibrium temperature than a simple application of Planck’s law would suggest... However, our findings are in perfect agreement with the more general theory of fluctuational electrodynamics, which allows one to take the geometry and the size of the body into account."[5]
This research will be useful in calculations involving the affect of
atmospheric aerosols on
climate, since
soot particles are nanoscale.[5]
References:
- Joshua J. Cardiel, Alice C. Dohnalkova, Neville Dubash, Ya Zhao, Perry Cheung and Amy Q. Shen, "Microstructure and rheology of a flow-induced structured phase in wormlike micellar solutions," Proc. Natl. Acad. Sci., vol. 110, no. 18 (April 30, 2013), pp. E1653-E1660 .
- Michelle Ma, "New device could cut costs on household products, pharmaceuticals," Washington University Press Release, April 12, 2013.
- Rainbow of Nanoparticle Colors is 'Hot' Development from Xu Research Group , Old Dominion University Press Release, October 7, 2010.
- C. Wuttke and A. Rauschenbeutel, "Thermalization via Heat Radiation of an Individual Object Thinner than the Thermal Wavelength," Phys. Rev. Lett. vol. 111, no. 2 (July 12, 2013), Document No. 024301 (2013) [5 pages].
- Florian Aigner, "Heat Radiation of Small Objects: Beyond Planck's Equations," Vienna University of Technology Press Release No. 60/2013, July 8, 2013.
Permanent Link to this article
Linked Keywords: Nanoscopic scale; nanoscale; physics; chemistry; nanoparticle; ratio; surface; atom; wavelength; light; powder; liquid; oil; water; hydrophilic; lipophilic; surfactant; molecule; research; University of Washington (Seattle, Washington); Pacific Northwest National Laboratory (Richland, Washington); experiment; Proceedings of the National Academy of Sciences; boundary layer; flow boundary; micrometer; deformation; cetrimonium bromide; cetyltrimethylammonium bromide; viscoelasticity; viscoelastic; sodium salicylate; shear-thinning; strain rate; micelle; micellar; Helmholtz free energy; energetically favorable; scanning electron microscope; scanning electron micrograph; Environmental Molecular Sciences Laboratory of the University of Washington; viscosity; National Science Foundation; macroscopic scale; chemical reaction; chemical process; heterogeneous catalysis; optics; optical; color; coloration; metal; Physical Review Letters; physicist; Vienna Center for Quantum Science and Technology; Vienna University of Technology (Vienna, Austria); temperature; thermal; absorption of electromagnetic radiation; optical absorber; radiative equilibrium; equilibrium state; Planck radiation law; electromagnetic radiation; spectral radiance; absolute temperature; Boltzmann constant; Planck constant; speed of light; blackbody curve; Max Planck; quantum mechanics; star; charcoal briquettes; room temperature; electrical engineering; transmission; radio reception; radio wave; antenna; first principles; silicon dioxide; silica; nanometer; infrared; thermal wavelength; A picture is worth a thousand words; Arno Rauschenbeutel; quantum electrodynamics; geometry; particulate; atmospheric aerosol; climate; soot.