Carbyne
August 28, 2013
One of the first things you learn about
organic chemistry is the difference between an
-ane, an
-ene, and an
-yne. The "anes," as typified by
ethane (CH3-CH3), are
molecules with a
single bond between
carbon atoms; the "enes," as typified by
ethylene (CH2=CH2), are molecules with a
double bond between carbon atoms; and the "ynes," as typified by
ethyne (CH≡CH, also called
acetylene, as if things weren't confusing enough), are molecules with a
triple bond between carbon atoms.
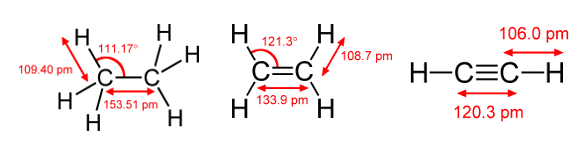
Carbon bonding in ethane, ethylene and acetylene (ethyne). Source images, from Wikimedia Commons, ethane, ethylene, and acetylene (ethyne))
As expected, a greater number of bonds results in a higher
bond-dissociation energy, as the following table shows.
One thing we notice is that the
energy is not
linear with bond number; otherwise, we would expect the bond-dissociation energy for a carbon-carbon triple bond to be 1041 kJ/mole, which is 24% higher than the actual value. This triple bond can be considered to be the
combination of a
sigma bond (369 kJ/mole) and two
pi bonds (268 and 202 kJ/mole).
Carbon nanotubes and
graphene are composed of bonded carbon atoms. Although there are just single bonds connecting their carbon atoms, the
regular arrangement of the atoms offers considerable strength in the assemblage. As a result, the
Young's modulus of single-walled carbon nanotubes is about a
terapascal (TPa), and that of single layer graphene is 2.4 ± 0.4 TPa.[1]
An interesting alternative to carbon nanotubes might be
braided strings of carbon atoms bonded together as in acetylene. This
linear acetylenic carbon, is a
carbon allotrope. It's also called carbyne. The structure of such a carbon chain would be written as ...C≡C-C≡C-C≡C..., although the reality would have all carbons joined by an
sp hybridized bond, as shown in the figure.
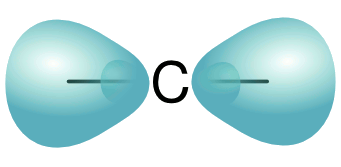
Illustration of sp hybridized bonds of a carbon atom.
(Modified Wikimedia Commons image by J.F. Melero.)
Carbyne is estimated to have a Young's modulus that's many times that of
diamond. The Young's modulus of diamond is 1.22 TPa. The
spectroscopic signature for carbyne has been detected in
interstellar space, and there have been attempts to
synthesize carbyne.[2-3] The products of most such syntheses appear to have been nanotubes, instead, so there's no
consensus on whether there has been a synthesis. One believable synthesis was for a 44-atom chain in
solution.[3] Some
chemists have calculated that carbyne is very unstable, and two strands of carbyne would
explosively react if they contacted each other.[3]
Betting on the idea that carbyne will eventually be synthesized in useful quantities, scientists from
Rice University (Houston, Texas) have performed
first-principles computer calculations of the properties of carbyne. Their calculations confirm the excellent
mechanical properties of this
material. One interesting property is the transition of bond type under
tensile stress, as shown in the figure.
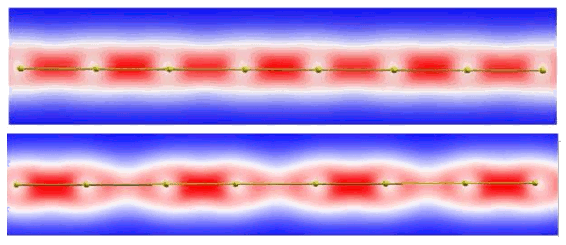
The carbyne bonds do not merely stretch under tensile strain. There's a transition to an alternating bond type that's more like an alternation of single and double bonds than acetylene hybrid bonds. (Fig. 1 of ref. 2, via the arXiv Preprint Server.)[2)]
Bending stress is better handled, as shown in the
electron density plot of the next figure.

Electron density plot of a string of carbyne atoms bent into a small radius.
(Fig. 2a of ref. 2, via the arXiv Preprint Server.)[2)]
The following table summarizes the calculated properties of carbyne.
The
persistence length is a measure of the
rigidity of a piece of fiber. This is the length scale over which portions of the fiber all point in the same direction. For comparison, the Young's modulus of diamond and graphene are 1.22 TPa and 2.4 TPa, respectively, and the shear modulus of diamond is 0.5 TPa. Carbyne outperforms these materials by more than an
order of magnitude.
One other interesting finding is the potentially large change in
bandgap with tension, changing from 3.2
eV to 4.4 eV with 10% strain.[2] It's also nice to know that carbyne isn't self-annihilating, since the calculations show a 0.6 eV barrier for
cross-linking of carbyne strings. This energy is equivalent to an
equilibrium cross-linking of once per 17 atoms, or 2.2
nm.[2]
References:
- Jae-Ung Lee, Duhee Yoon and Hyeonsik Cheong, "Estimation of Young's Modulus of Graphene by Raman Spectroscopy," Nano Lett., vol. 12, no. 9 (August 6, 2012), pp 4444-4448.
- Mingjie Liu, Vasilii I. Artyukhov, Hoonkyung Lee, Fangbo Xu and Boris I. Yakobson, "Carbyne from first principles: Chain of C atoms, a nanorod or a nanorope?" arXiv Preprint Server, August 9, 2013.
- New Form of Carbon is Stronger Than Graphene and Diamond, The Physics arXiv Blog, MIT Technology Review, August 15, 2013.
Permanent Link to this article
Linked Keywords: Organic chemistry; ethane; molecule; carbon-carbon bond; single bond; carbon; atom; ethylene; double bond; ethyne; acetylene; triple bond; Wikimedia Commons; bond-dissociation energy; Joule; kJ; mole; -ane; -ene; -yne; energy; linearity; linear; molecular orbital theory; sigma bond; pi bond; carbon nanotube; graphene; crystal structure; regular arrangement; Young's modulus; pascal; terapascal; Tpa; braid; fiber; string; linear acetylenic carbon; carbon allotrope; sp hybridized; bond; diamond; spectroscopy; spectroscopic signature; interstellar medium; interstellar space; chemical synthesis; synthesize; scientific consensus; solution; chemist; explosive material; explosively react; Rice University (Houston, Texas); first-principles; computer simulation; computer calculation; strength of materials; mechanical property; material; tensile stress; tensile strain; arXiv Preprint Server; electron density; plot; radius; Shear modulus; Poisson's ratio; persistence length; kelvin; K; nanometer; nm; stiffness; rigidity; order of magnitude; bandgap; electronvolt; eV; cross-link; cross-linking; thermodynamic equilibrium.