Tough Gel
September 12, 2012
As a
child, one of my favorite
toys was my set of
Lincoln Logs. In those dim days before
electronics, toys were very simple; and they could be sold, unchanged, for decades. Lincoln Logs are a good example of this, since they are just notched pieces of
wood (see figure). They were
patented in 1920 by
John Lloyd Wright, a son of the
Fallingwater architect,
Frank Lloyd Wright.
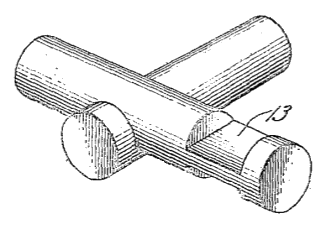
Lincoln Logs
Figure five of US Patent No. 1,351,086, "Toy Cabin Construction," by J. L. Wright (August 31, 1920).
(Via Google Patents).[1)]
John was an architect like his father, and there's an important architectural lesson to be learned from Lincoln Logs. Interlocking members offer great
structural strength. This was a lesson I subconsciously learned in my play, and it's a good reason to give your children toys like Lincoln Logs and
Erector Sets. I guess the modern equivalents would be
Lego, which now include
robotic sets. Yes, the present world is a
computerized world, and there's no escape from it.
A team of
scientists from the
Harvard University School of Engineering and Applied Sciences have used this interlocking approach on a
molecular level to produce an especially
tough gel. The gel is an interlocking structure of two gels,
polyacrylamide gel and
alginate gel (see figure). The structure has the desirable mechanical property that
stress is distributed over a wide area, thereby mitigating
rupturing forces that appear at
crack tips.[2-3]
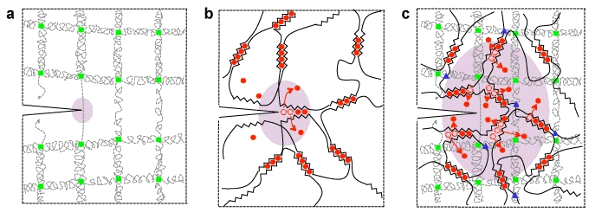
Structure of polyacrylamide gel (a), alginate gel (b), and a combined hydrogel (c). Calcium ions are shown as red circles, with blue triangles and green squares showing covalent crosslinks between chains. The combined structure spreads stress from a flaw, such as a crack, over a wider area (purple).(Harvard SEAS image/Jeong-Yun Sun and Widusha R. K. Illeperuma).[2)]
The
interdisciplinary team has expertise in
mechanics,
materials science, and
tissue engineering, and one intended application for this gel is as a
biocompatible replacement for damaged
cartilage in
human joints.[2] The essential biocompatibility is aided by the fact that these
hydrogels are about 90%
water.[3] A summary of this research appears in the September 6, 2012, issue of
Nature.[3]
Conventional hydrogels don't have much mechanical strength, otherwise it would be difficult to eat your
gelatin dessert. The new, mechanically strong hydrogel combines two well known hydrogels. Polyacrylamide is used to make soft contact lenses, and it's also useful as an
electrophoresis gel. Alginate, as its name indicates, is a seaweed extract. Alginate is used as a
food thickening agent.
These two component hydrogels are individually weak. Alginate can only endure a 120%
elongation before breaking. The composite gel, which is mixed in an 8:1 ratio of the polyacrylamide to alginate, develops
crosslinks between the component gels to impart strength. The polyacrylamide chains, arrayed in a grid-like structure
bond covalently with the alginate chains. Some
calcium ions are added to the water solution to enhance bonding.[2]
Thin films of this hydrogel withstand an elongation of 2100% before rupture. Other hydrogels have achieved elongations nearly as large, but they lack
fracture toughness; that is, they fail easily if a notch or other flaw is present. Cartilage has a fracture
energy of about 1,000
J m−2, whereas most hydrogels have a fracture energy of just 10 J m
−2. Some synthetic gels have fracture energies of 100–1,000 J m
−2. This new gel has a fracture energy of about 9,000 J m
−2.[3]
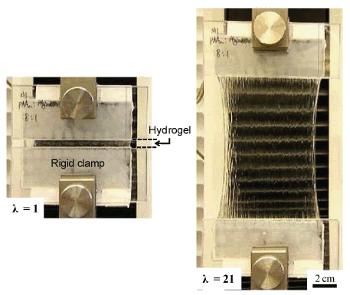
A thin sheet of the tough gel can be stretched to 2100% elongation before breaking.
(Harvard SEAS image/Jeong-Yun Sun).[2)]
The stretching is repeatable, provided that a
relaxation time is allowed between stretches.
This research was funded by the
U.S. Army Research Office, the
National Science Foundation, the
Defense Advanced Research Projects Agency and the
National Institutes of Health. The National Science Foundation also provides support for the
Harvard Materials Research Science and Engineering Center.[2]

Follow the bouncing ball.
A picture is worth a thousand words, as this image of a steel ball impacting the gel demonstrates.
(Still image from a Harvard SEAS video).[4)]
![]()
References:
- J. L. Wright, "Toy Cabin Construction," US Patent No. 1,351,086, August 31, 1920
- Tough gel stretches to 21 times its length, recoils, and heals itself, Harvard School of Engineering and Applied Sciences Press Release, September 5, 2012.
- Jeong-Yun Sun, Xuanhe Zhao, Widusha R. K. Illeperuma, Ovijit Chaudhuri, Kyu Hwan Oh, David J. Mooney, Joost J. Vlassak and Zhigang Suo, "Highly stretchable and tough hydrogels." Nature, vol. 489, no. 7414 (September 6, 2012), pp. 133-136.
- Video showing a metal ball dropping onto a very thin sheet of hydrogel without rupturing it, Harvard School of Engineering and Applied Sciences video (15 MB MP4), September 5, 2012.
Permanent Link to this article
Linked Keywords: Child; toy; Lincoln Logs; electronics; wood; patent; John Lloyd Wright; Fallingwater; architect; Frank Lloyd Wright; Google Patents; shear strength; structural strength; Erector Set; Lego; robotic set; embedded system; computerized; scientist; Harvard University; School of Engineering and Applied Sciences; molecule; molecular; fracture toughness; tough; gel; polyacrylamide; alginate; stress; ductile fracture; rupture; stress intensity factor; crack tip; hydrogel; calcium; ion; covalent bond; covalent crosslink; Jeong-Yun Sun; Widusha R. K. Illeperuma; interdisciplinarity; interdisciplinary; mechanics; materials science; tissue engineering; biocompatibility">biocompatible; cartilage; human joint; water; Nature; gelatin dessert; electrophoresis gel; food thickening agent; elongation; crosslink; covalent bond; energy; joule; J; meter; m; relaxation; relaxation time; U.S. Army Research Office; National Science Foundation; Defense Advanced Research Projects Agency; National Institutes of Health; Harvard Materials Research Science and Engineering Center.