Tin Pest and Purple Plague
April 24, 2012
The development of
integrated circuitry was not without its surprises, and it offered new research opportunities for my fellow
materials scientists. One problem involved the connection of the
electrical signals from the
silicon chips to the outside world.
Nowadays,
copper is used as the
conductor in high density integrated circuits to route
voltage and
current between
transistors and other circuit elements. This
copper Damascene process is replacing the earlier technology in which
aluminum was used for these on-chip connections. The connection to the package terminals is done with thin, highly conductive and very flexible
gold wires.
Bonding of the gold wires to the aluminum can be done by pressing a heated gold wire on the aluminum bonding pad with a little rubbing provided by an
ultrasonic excitation of the bonding
ferrule. Pull testing will prove that the connection is mechanically strong, as will proper
electrical conductance of the joint.
The problem in the gold-aluminum bond happens when too much heat is used to make the connection. In those cases, you end up with a low conductance joint characterized by either a
white or
purple coloration. These problems were poetically named the white and purple
plagues. An illustration of a purple plague connection of gold to aluminum is shown in the figure.
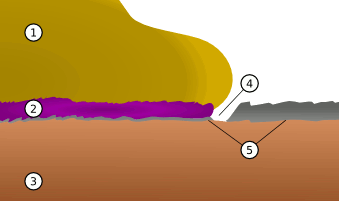
A purple plague join (2) between gold (1) and aluminum (5) on a silicon chip (3). Growth of the AuAl2 intermetallic can cause a void in the aluminum (4).
(Via Wikimedia Commons))
These "plagues" are actually
intermetallic compounds that form between the gold and aluminum. Purple plague is the AuAl
2 intermetallic, and the white plague is the Au
5Al
2 intermetallic. As their name implies, intermetallic compounds have properties somewhat like
compounds and less like
alloys. Their properties can be quite unlike that of compositions of slightly different proportions of the same
elements. The gold-aluminum
phase diagram is shown below.
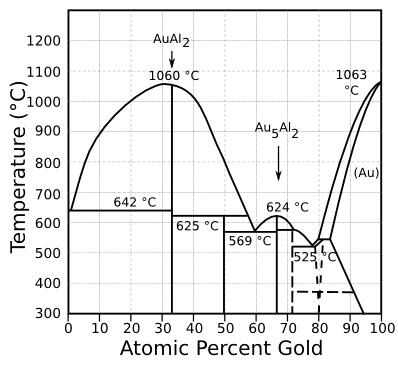
The gold-aluminum phase diagram.
The intermetallic compounds, AuAl2 (purple plague) and Au5Al2 (white plague) are shown.
(Modified Wikimedia Commons image))
These plagues are prevented by keeping the bonding
temperature low, so the intermetallic phase is not
thermodynamically favored. This is accomplished by using less heat and more ultrasonic rubbing.
These plagues happen at elevated temperature, but there's an interesting process for
tin that happens at ordinary temperatures. This isn't a plague, it's a pest,
tin pest. Tin pest is a deterioration of tin caused by a
phase transformation of the pure element at 13.2
°C (about 56
°F). Above this temperature, tin is the silvery,
ductile metal we usually see, but below this temperature, it's brittle,
grey, and nonmetallic.
Phases are labeled by
Greek letters in
alphabetic order from low temperature to high, so the low temperature form is called α-tin, and the higher temperature form is called β-tin. Fortunately, this transformation has a high
activation energy, so it proceeds very slowly at the start. The initial phase, however, "
seeds" subsequent transformation, so it's a propagating transformation. The
volume increase caused by the phase change causes a tin body to crumble into an α-tin powder. At very low temperatures, this disintegration is rapid and complete.
Tin pest was noted in early
church organ pipes. These were sometimes made of
silver, but mostly
zinc, probably as a mitigation against tin pest. They say that
an army marches on its stomach, but it travels best when fully clothed. There's a
theory that
Napoleon's army was defeated during its
Russian campaign because tin pest caused it to lose its tin
buttons. Fortunately, what we call "
tin cans" are actually made from
coated steel.

Pipe organ at St. Stephan's Cathedral, Passau, Germany.
This organ has 17,774 pipes.
Some early organ pipes were made from tin, an inexpensive and readily available metal. The climate in northern Europe was such that they were attacked by tin pest.
(Via Wikimedia Commons))
Tin pest would have remained just an interesting tale in the history of
metallurgy, were it not for recent efforts to remove
lead from
solder. The
European Restriction of Hazardous Substances Directive (RoHS), and directives in other countries, have caused a problem to electronic circuit manufacturers, since the low melting point lead-tin
eutectic solder can no longer be used. In some cases, pure tin has been used, leading to tin pest problems.[1]
Lead-free solders use alloying elements, such as
antimony or
bismuth, silver and
indium, to both lower the melting point and prevent tin pest.[2]
One interesting tin pest problem was published on
arXiv by
scientists at the
Laboratoire National des Champs Magnétiques Intenses,
Grenoble, France.[3]
Superconducting magnets need to cycle to very low temperatures, and it was found that joints made with the Sn
96Ag
4 lead-free solder had a 37% lower
shear rupture strength at 77K than those made from the standard Sn
60Pb
40 solder. They attribute the problem to tin pest.
For the
tin man's sake, we can only hope that
Oz maintains an
idyllic temperature at all
seasons of the year.[4]
References:
- Michael Barthelmy, "Problems With Pure Tin Coatings," NASA, January 17, 2001.
- Kenneth A. LaBel and Michael J. Sampson, "Developing a NASA Lead-free Policy for Electronics - Lessons Learned," TRISMAC 2008 Conference, April 15, 2008.
- R. Pfister and P. Pugnat, "Tin Pest: A Forgotten Issue in the Field of Applied Superconductivity?" arXiv Preprint Server, April 6, 2012.
- The Wizard of Oz, 1939, Victor Fleming, George Cukor, Mervyn LeRoy, Norman Taurog and King Vidor, Directors, Internet Movie Database.
Permanent Link to this article
Linked Keywords: Integrated circuit; materials scientist; materials science; electrical signal; silicon chip; copper; conductor; voltage; current; transistor; copper Damascene process; aluminum; gold; wire bonding; ultrasonic; ferrule; electrical conductance; white; purple; plague; Wikimedia Commons; intermetallic compound; chemical compound; alloy; element; phase diagram; temperature; Gibbs free energy; thermodynamically favored; tin; tin pest; phase transition; phase transformation; Celsius; °C; °F; ductility; metal; grey; Greek alphabet; collation; alphabetic order; activation energy; seed crystal; volume; church organ; pipe organ; silver; zinc; an army marches on its stomach; theory; Napoleon; Invasion of Russia; button; tin can; coated steel; St. Stephan's Cathedral, Passau, Germany; northern Europe; metallurgy; lead; solder; European Union; Restriction of Hazardous Substances Directive; RoHS; eutectic; lead-free solder; antimony; bismuth; indium; arXiv; scientist; Laboratoire National des Champs Magnétiques Intenses; Superconducting magnet; shear rupture strength; Tin Woodman; tin man; The Wizard of Oz; Oz; idyllic; seasons of the year.