Tellurium - A Stellar Material
February 29, 2012
Many
materials scientists, myself included, have worked with
tellurium, a rare
chemical element with
atomic number 52. My application was
thermopower energy-harvesting.
Bismuth telluride (Bi
2Te
3) and its close cousins are the star materials for this application, but tellurium is useful in so many ways.
Mercury cadmium telluride and
lead telluride are excellent infrared detectors.
Cadmium telluride is a useful
photovoltaic material. You may even have tellurium in your home, since its
oxide is used as a
phase-change material in
rewritable optical discs.
Tellurium glasses, such as Ge
2Sb
2Te
5, can be used as the active material in
phase change memory, a technology I mentioned in two previous articles (
Nano Memory, September 30, 2010 and
Phase Change Conductance, June 21, 2011).
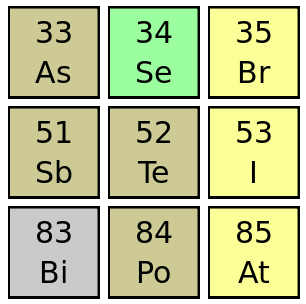
Tellurium and its neighbors in the Periodic Table.
When younger, I was confused as to why antimony should be called Sb.
This derives from its Latin name, "stibium."
(Via Wikimedia Commons))
Well, this "star" material has now been confirmed as a "stellar material." A team of
astrophysicists with members from many US institutions has discovered neutral tellurium in three so-called
metal-poor stars. This discovery was made using the
Hubble Space Telescope, managed by the
Space Telescope Science Institute. Details are published in a recent issue of
The Astrophysical Journal Letters.[1-4]
One self-referential aspect of materials science is that materials scientists are themselves made from materials. As is well known to all with even a passing interest in astronomy, we are all made from "star stuff." The universe stared out with mostly
hydrogen, some
helium, and a little
lithium; but
nucleosynthesis reactions within stars manufactured all the other elements of the periodic table.
The
first populations of stars had few heavy elements. Subsequent stars were created from material synthesized in these first stars, so they have more heavy elements. Since the
Sun is from a later generation of stars,
Earth has the panoply of elements that we see.
Nucleosynthesis of heavier elements is hard to do, and that's why these elements are rare.
Supernovae perform what's called
r-process nucleosynthesis to create neutron-rich nuclei. The
r in r-process stands for rapid, since this type of nucleosynthesis proceeds by a rapid succession of neutron capture on a lighter seed nucleus. Not surprisingly, there's a companion slow process, the
s-process. About half the heavier elements are thought to have been created by the s-process, and the rest by the r-process.
Tellurium is a rare element with a
crustal abundance of just 1 μg/kg, or a
part-per-billion. The rarest of the so-called
rare earth elements have crustal abundances that are hundreds of times greater.
The
isotopes of tellurium are quite interesting. The
stable isotopes of tellurium (
122Te,
124Te,
125Te and
126Te) comprise just a third of natural tellurium. The rest is
radioactive isotopes with extremely long
half lives. Although technically radioactive,
128Te has a half life of 2.2 x 10
24 years, which is more than a hundred trillion times the
age of the universe.[5]
The Hubble Telescope observation is the first time that tellurium has been detected in stars. Tellurium was detected in three stars, designated
BD +17 3248, HD 108317, and HD 128279. These are ancient stars, nearly 12 billion years old, located a few thousand
light-years away.[1-2]
The tellurium was detected using near-
ultraviolet spectroscopy, and the ratio of tellurium to the heavy elements,
barium and
strontium, was the same for all three stars. This supports the theory that a rare type of supernova was responsible for the creation of the very heavy elements, including tellurium.[3]
Anna Frebel, an
assistant professor of physics at MIT, and a co-author of the tellurium paper, had this to say:
"You can make iron and nickel in any ordinary supernova, anywhere in the universe... But these heavy elements seem to only be made in specialized supernovas. Adding more elements to the observed elemental patterns will help us understand the astrophysical and environmental conditions needed for this process to operate."[3]
Home, Sweet Home
Artist's impression of our Milky Way Galaxy.
(NASA image, via Wikimedia Commons))
References:
- Ian U. Roederer, James E. Lawler, John J. Cowan, Timothy C. Beers, Anna Frebel, Inese I. Ivans, Hendrik Schatz, Jennifer S. Sobeck and Christopher Sneden, "Detection of the Second R-Process Peak Element Tellurium in Metal-Poor Stars," The Astrophysical Journal Letters, vol. 747, no. 1 (March 1, 2012), doi:10.1088/2041-8205/747/1/L8.
- Ian U. Roederer, James E. Lawler, John J. Cowan, Timothy C. Beers, Anna Frebel, Inese I. Ivans, Hendrik Schatz, Jennifer S. Sobeck and Christopher Sneden, "Detection of the Second r-process Peak Element Tellurium in Metal-Poor Stars," arXiv Preprint Server, February 10, 2012.
- Jennifer Chu, "Rare Earth element found far, far away," MIT Press Release, February 17, 2012.
- Usman Zafar Paracha,"Tellurium, Rare Earth Element, Observed in Three Ancient Stars," Technorati, February 21, 2012.
- Tellurium page on Wikipedia.
Permanent Link to this article
Linked Keywords: Materials scientists; tellurium; chemical element; atomic number; thermopower; energy-harvesting; bismuth telluride; mercury cadmium telluride; lead telluride; cadmium telluride; photovoltaic material; tellurium dioxide; phase-change; rewritable optical disc; tellurium glass; phase change memory; Periodic Table; antimony; Wikimedia Commons; astrophysicist; metal-poor star; Hubble Space Telescope; Space Telescope Science Institute; The Astrophysical Journal Letters; hydrogen; helium; lithium; nucleosynthesis; metallicity; first populations of stars; Sun; Earth; supernova; r-process; s-process; crustal abundance; part-per-billion; rare earth element; isotopes of tellurium; stable isotope; radionuclide; radioactive isotope; half life; age of the universe; BD +17 3248; light-year; ultraviolet; spectroscopy; barium; strontium; Anna Frebel; assistant professor of physics; iron; nickel; Milky Way Galaxy; NASA.