Oxygen-Enriched Combustion
May 28, 2012
My first experience with
gas-permeable membranes was as a young child. In those days, before
mylar was used for this purpose,
helium balloons were made from
rubber. I found that my balloon became deflated after just a day. Anlayzing the problem, I decided that the neck hadn't been tied properly, and I decided that the next time I would tie my own, second knot to prevent escape of the
gas.
Well, that didn't work, since my
hypothesis was wrong. Of course, at age five I knew nothing about the element, helium, or the properties of rubber. Later, I was using helium for
leak testing of
vacuum systems. Helium is inert, it's a
monoatomic gas, and it's also the smallest gas molecule. For these reasons, helium leaks through almost everything.
Polymers other than rubber have been engineered to be
specifically permeable to gas molecules smaller than a particular size. These are used for
gas separation, one application for which is
fuel tank inerting. These membranes, used in
nitrogen generators, separate
nitrogen and
oxygen from
air so that only nitrogen exists in the empty space (
ullage) of fuel tanks, for
obvious safety reasons.
Although
polymer membranes are useful at low
temperatures, they will decompose at high temperatures. Fortunately, polymers are not the only materials that act as separators. Some
ceramics will work as well.[1] A team of
engineers at
MIT, led by
Ahmed F. Ghoniem, the Ronald C. Crane Professor of
Mechanical Engineering, has been investigating ceramic membranes as a way to separate oxygen from the air as a means of doing cleaner
combustion of
fossil fuels.[2-4]
Danger!
Hot Technology.
Members of
Ahmed F. Ghoniem's
lab at MIT.
Left to right, Anton Hunt, Ahmed Ghoniem, Patrick Kirchen and James Hong.
(MIT Photo).[2)]
When combustion of fuels is done with air, a gas that's about three-quarters nitrogen, small quantities of
nitrogen dioxide (NO
2) are produced, along with the expected
carbon dioxide and
water vapor. One
global greenhouse mitigation method is
carbon sequestration, in which the CO
2 is captured and "buried" underground. It would be nice if the effluent gas going into the carbon sequestration process didn't contain all that nitrogen.
Burning fuels in pure oxygen, and not air, is called oxyfuel combustion. The MIT engineering team has done previous work in
pressurized oxyfuel combustion, a process that improved
fuel efficiency. Their current research is in the development of a ceramic membrane process so this technology can be used in future and existing
powerplants as a way to reduce greenhouse gases.[2] Says team leader, Ahmed Ghoniem,
"The whole objective behind this technology is to continue to use cheap and available fossil fuels, produce electricity at low price and in a convenient way, but without emitting as much CO2 as we have been."[2]
The MIT membrane process is pictured, below.[4]
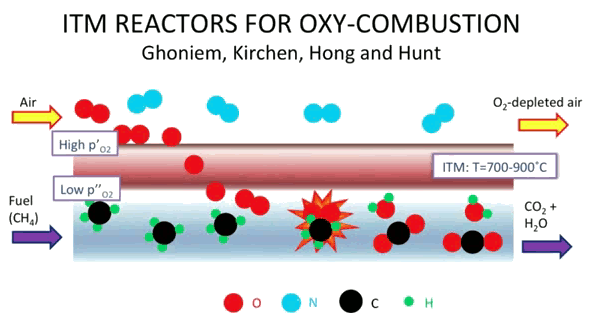
The MIT oxygen membrane combustion process. Oxygen molecules (red) and nitrogen molecules (blue) from air pass over the ceramic membrane, and only oxygen passes through to react with carbon (black) and hydrogen (green). The resultant carbon dioxide product can be captured and stored. (Screen capture from a YouTube video).[4)]
Readers might remember
Le Chatelier's principle from their
high school or
college freshman chemistry courses. One expression of this principle, which is actually a qualitative statement of the affect of
reactant and
product concentrations on the
rate constant, is that removal of products leads to a faster
reaction. In the MIT combustion membrane process, the combustion removes oxygen from one side of the membrane, allowing more oxygen to pass through. Says Ghoniem,
"It turns out to be a clever way of doing things... The system is more compact, because at the same place where we do separation, we also burn. So we’re integrating everything, and we’re reducing the complexity, the energy penalty, and the economic penalty of burning in pure oxygen and producing a carbon dioxide stream."[2]
Aside from experiments on various temperatures, pressures and fuels, the MIT team is developing a
computational model for their process. One interesting result is that gas flow protects the material when the gas temperature exceeds material limits.[2-3]
References:
- See, for example, Renate M. de Vos and Henk Verweij, "High-Selectivity, High-Flux Silica Membranes for Gas Separation," Science, vol. 279, no. 5357 (March 13, 1998) pp. 1710-1711.
- Jennifer Chu, "Oxygen-separation membranes could aid in CO2 reduction," MIT Press Release, May 21, 2012.
- Jongsup Hong, Patrick Kirchen, and Ahmed F. Ghoniem, "Numerical simulation of ion transport membrane reactors: Oxygen permeation and transport and fuel conversion," Journal of Membrane Science, vol. 407-408, July 15, 2012, pp. 71-85.
- Oxygen-separation membranes could aid in CO2 reduction, YouTube video, May 11, 2012.
Permanent Link to this article
Linked Keywords: Gas-permeable membrane; BoPET; mylar; helium; balloon; rubber; gas; hypothesis; leak detection; leak testing; vacuum system; monoatomic gas; semipermeable membrane; permeable; gas separation; fuel tank inerting; nitrogen generator; nitrogen; oxygen; air; ullage; TWA Flight 800; safety; polymer; membrane; temperature; ceramic; engineer; Massachusetts Institute of Technology; MIT; Ahmed F. Ghoniem; Anton Hunt; Patrick Kirchen; James Hong; mechanical engineering; combustion; fossil fuel; nitrogen dioxide; carbon dioxide; water vapor; global greenhouse; carbon sequestration; pollution; pressure; pressurized; fuel efficiency; power station; powerplant; YouTube; Le Chatelier's principle; high school; college freshman; chemistry; reactant; product; concentration; rate constant; reaction; computational model.