Flow Energy Storage
December 12, 2012
The principal problem of most
renewable energy sources is that their
energy supply is not constant.
Hydroelectric and
tidal power plants are renewable energy sources with fairly constant output, but you can't get
solar energy at night, and you can't get too much of it on cloudy days.
Wind turbines will supply power at night, but only when the wind blows. You need to store energy so it will be available for use later.

Nineteen megawatts peak by day, but zero megawatts at night.
A photovoltaic system near Thüngen, Bavaria, Germany.
(Photograph by OhWeh, via Wikimedia Commons)
In a
previous article (Flow Batteries, July 18, 2012), I reviewed the flow
capacitor, an interesting energy storage technology for
load balancing in
renewable energy systems. A flow capacitor stores
electric charge in a fluid from which power can be later extracted. The flow capacitor is actually a variation of a similar device called a
flow battery.
A standard
electrochemical battery stores
energy in the
potential energy of the
chemical reactions it contains. In most batteries, the
electrodes participate in these reactions, but there are some reactions that involve only the
electrolyte solutions, so the electrode materials are not consumed. In a flow battery, you can separate the
charge/discharge portion from the energy storage portion. Your energy can be stored in a storage tank.
Scientists at
Harvard University's School of Engineering and Applied Sciences are developing flow battery systems under an innovation grant from the
U.S. Department of Energy through its
Advanced Research Projects Agency–Energy (ARPA-E) program. ARPA-E is presently funded at $130 million to advance innovative energy technologies.[1]
The Harvard team, led by
Materials Science professor,
Michael Aziz, previously developed a
hydrogen-
chlorine regenerative fuel cell, which can function also as a flow battery (see figure).[2] Chlorine, of course, is a difficult chemical. It will even dissolve
platinum, a common electrode material, by forming
chloroplatinic acid (H
2PtCl
6). The
HCl system was chosen over the typical hydrogen-
oxygen system of fuel cells, since the chlorine system is much more efficient, the principal reason being that the chlorine reaction involves just a single
electron.[3]
This HCl cell used a
carbon electrode coated with a (
Ru0.09Co0.91)
3O
4 catalyst at the chloride side, and a platinum electrode at the hydrogen side. The
proton exchange membrane (PEM), typically
Nafion, conducts
H+ ions (generally called, but not precisely,
protons) in both directions.
The peak
power density was greater than a
watt per
square centimeter of electrode area, and the cell could be run with 90%
efficiency at 0.4 W/cm
2.[2] It was possible to produce cells having less than 0.15 milligrams of Ru per square centimeter of electrode area;[2] but the presence of the expensive metals, Ru and Pt, along with the corrosive nature of chlorine, would be impediments in commercial use of such cells.
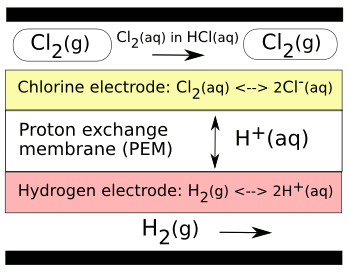
A hydrochloric acid (HCl) regenerative fuel cell/flow battery. HCl is electrolyzed by a voltage to form Cl2 on the anode side of the cell, and H2 on the cathode side. In reverse mode, reaction of the gases regenerates HCl and gives a voltage.
(Image by the author, rendered with Inkscape).[2)]
The key to a commercial flow battery is its electrolyte system, and development of a better chemical system is what the Harvard team intends to do. They are collaborating with
Sustainable Innovations, LLC,, a commercial electrochemical system developer.[1] Their focus is on an undisclosed class of small
organic molecules that are
non-toxic and inexpensive.[1] The current class of flow batteries have problematic chemical systems.
One
type of flow battery uses
vanadium, but vanadium is an expensive metal, selling for more than twenty-five times the price of
iron.
Sodium-sulfur is another flow battery chemical system, but high
temperature is required to keep the components in their
molten state, and the components are
corrosive. All this adds cost and complexity. The type of flow battery envisioned by the Harvard team is one that could be placed in a home to locally buffer solar energy.[1]
The US Department of Energy has a serious commitment to battery technology. It's set a 5/5/5 goal for the development of batteries within five years that have five times greater storage capacity at a fifth the price, and it's created a
Joint Center for Energy Storage Research at
Argonne National Laboratory. This center will be funded at $120 million over its first five years, and it will have the mix of scientists and
engineers that made
research and development organizations, such as
Bell Labs, so effective in the past.[4]
References:
- Greener storage for green energy, Harvard School of Engineering and Applied Sciences Press Release, November 28, 2012.
- Brian Huskinson, Jason Rugolo, Sujit K. Mondal and Michael J. Aziz, "A High Power Density, High Efficiency Hydrogen-Chlorine Regenerative Fuel Cell with a Low Precious Metal Content Catalyst," arXiv Preprint Server. June, 13, 2012.
- Hydrogen-Chlorine Fuel Cell, Harvard University Web Site.
- Patrick Thibodeau, "DOE wants 5X battery power boost in 5 years," Computer World, November 30, 2012.
Permanent Link to this article
Linked Keywords: Renewable energy; energy; hydroelectricity; tidal power; solar energy; wind power; wind turbine; megawatt; photovoltaic system; Thüngen; Bavaria, Germany; Wikimedia Commons; capacitor; load balancing; electric charge; flow battery; electrochemical battery; potential energy; chemical reaction; electrode; electrolyte solution; charge cycle; charge/discharge; scientist; Harvard University; School of Engineering and Applied Sciences; U.S. Department of Energy; Advanced Research Projects Agency–Energy; Materials Science; professor; Michael Aziz; hydrogen; chlorine; regenerative fuel cell; platinum; chloroplatinic acid; hydrogen chloride; HCl; oxygen; electron; carbon; ruthenium; Ru; cobalt; Co; catalysis; catalyst; proton exchange membrane; Nafion; hydron; H+ ion; proton; power density; watt; square centimeter; efficiency; Inkscape; Sustainable Innovations, LLC; organic compound; organic molecule; toxicity; non-toxic; vanadium redox battery; vanadium; iron; sodium-sulfur battery; sodium; sulfur; temperature; molten; corrosive; Joint Center for Energy Storage Research; Argonne National Laboratory; engineer; research and development; Bell Labs.