Coal
July 23, 2012
As we work to develop
materials, we often overlook the fact that real world materials are quite unlike the 99.999% pure
chemicals we use in our
laboratories. When our new materials leave the laboratory and become production items, there are a myriad of things that can go wrong because the lesser grades of materials used to compound a product may have important
impurities. As an example,
sulfur is one impurity that leads to
corrosion problems with
high temperature superalloys.
We forget that nearly every
inorganic chemical we use is dug from the
ground and is somehow
refined to the point at which it contains just one, or a few,
elements. However, there are some things that are just dug from the ground and used as-is. One example is
kieselguhr, more commonly known as diatomaceous earth.

Nature Imitating Art
The diatom, Aulacodiscus Grevilleanus, as drawn by Ernst Haeckel (1834 - 1919).
Haeckel was an eminent German biologist and artist. He discovered and named thousands of species.
(Via Wikimedia Commons))
Diatomaceous earth consists of the
silica-rich
fossil shells of
diatoms, a type of
algae (See figure). The fossil remains are laid in thick
sediments, and they are composed of a useful mixture of silica (SiO
2, 80-90%),
alumina (Al
2O
3, 2-4%) and
iron oxide (Fe
3O
4, 0.5-2%). Diatomaceous earth is used, not surprisingly, as an
abrasive.
Diatomaceous earth is also used for
filtration, a
filler for
plastics, and a
support medium for
catalysts. Its
absorbency makes it useful for
cat litter, and that's how the
Nobel Prize and the
family cat have something in common.
Alfred Nobel made his fortune by mixing
nitroglycerin and diatomaceous earth to form
dynamite, a stable
explosive. As can be expected from its composition, diatomaceous earth is a good material for
thermally insulating high temperature
furnaces.
The most important material to be dug from the ground and used in its pristine state is
coal. Coal fueled the
Industrial Revolution, and about 45% of
electrical generation in the
US in 2009
was from coal. The use of coal, however, declined from 52.8% in 1997. The former prominence of coal-fired power plants is because coal is cheap. The current decline is because the burning of raw coal
damages the environment.
The environmental damage isn't just from the
carbon dioxide building up in the
atmosphere, it's from the impurities in coal. The
sulfur dioxide formed in
combustion of the sulfur impurity in coal causes
acid rain when converted to
sulfuric acid in the atmosphere. Coal also contains significant quantities of
mercury; so much so, that many
tons of mercury are injected into the atmosphere from coal combustion each year.
If it wasn't for coal, our lives would be quite different. So, what's the origin of coal? Coal is the
fossilized remains of
plants that lived about 300-360 million years ago.[1] What was fossilized was mostly
lignin, a complex
polymer that's a part of the
cell walls of plants. Lignin serves to give
wood its robust
mechanical properties. The deposition of coal came to an abrupt end at the aptly-named
Carboniferous period. Why did this happen?
A research study by a team of 71
scientists from twelve
countries,[2] funded by the
National Science Foundation and published in the June 29, 2012, issue of
Science,[3-4] suggests that
white rot fungi evolved at the end of the Carboniferous with the capacity to digest lignin.[2] White rot might be the factor that ended the sixty-million year span of coal deposition, since it may have single-handedly destroyed the accumulation of woody debris that would have fossilized as coal.[2]
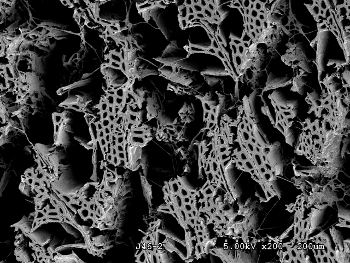
A scanning electron micrograph of wood that has been decayed by white rot. The rot has largely destroyed the wood structure.
(NSF photo, Robert A. Blanchette / Joel A. Jurgens, University of Minnesota))
David Hibbett, a
Professor of
Biology at
Clark University, led the research team.[2] Hibbett is an acknowledged expert on
fungi, and Clark University maintains the
Clark Fungal Database. The team looked at the
genomics of fungi known as
Basidiomycetes, which include white rot fungi.[1] They compared 31 fungal genomes, 26 of which were sequenced at the
Department of Energy's Joint Genome Institute.[2]
The team did a "
molecular clock" analysis of genome
mutations that traced the lignin-degrading ability back about 290 million years.[2] Says Hibbett,
"Our results suggest that the ability of fungi to break down lignin evolved only once."[2]
The same
organisms that ended the production of coal might be able to help with our future
energy requirements. The fungal genomes might offer the basis for development of fungal species for
biofuel production. These would break down lignin of woody plants, releasing
cellulose from cell walls that could be
fermented into
sugars. These sugars would then be used for production of
alcohol by
yeast for biofuel.[2] Fungi may also be used for
bioremediation, since they can break down complex
organic molecules.[2]
Says
Joseph Spatafora, a professor at
Oregon State University and a co-author on the study,
"There's an estimated 1.5 million species of fungi... We have names for about 100,000 species, and we're looking at 1,000 fungi in this project. This is still the tip of the iceberg in looking at fungal diversity and we're trying to learn even more to gain a better idea of fungal metabolism and the potential to harness fungi for a number of applications, including bioenergy. It's a really exciting time in fungal biology, and part of that is due to the technology today that allows us to address the really longstanding questions."[1]
References:
- Tracking the Remnants of the Carbon Cycle: How an Ancestral Fungus May Have Influenced Coal Formation, Joint Genome Institute Press Release, June 28, 2012.
- Study on Fungi Evolution Answers Questions About Ancient Coal Formation and May Help Advance Future Biofuels Production, NSF Press Release 12-117, June 28, 2012.
- Chris Todd Hittinger, "Endless Rots Most Beautiful," Science, vol. 336 no. 6089 (June 29, 2012) pp. 1649-1650.
- David S. Hibbett, et al., "The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes," Science, vol. 336 no. 6089 (June 29, 2012) pp. 1715-1719.
- The Hibbett Laboratory at Clark University.
- David Hibbett, "Evolutionary Perspectives on Diversity of Lignocellulose Decay Mechanisms in Basidiomycetes" at the 7th Annual Genomics of Energy & Environment Meeting on March 21, 2012 in Walnut Creek, CA.
Permanent Link to this article
Linked Keywords: Material; chemical compound; chemical; laboratory; impurity; sulfur; corrosion; high temperature; superalloy; inorganic chemical; lithosphere; ground; refining; refined; element; kieselguhr; Ernst Haeckel (1834 - 1919); German; biologist; artist; species; Wikimedia Commons; silica; fossil; shell; diatom; algae; sedimentary rock; sediment; alumina; iron oxide; abrasivevfiltration; filler; plastic; catalyst support; support medium; catalyst; absorbency; cat litter; Nobel Prize; family cat; Alfred Nobel; nitroglycerin; dynamite; explosive; thermal insulation; furnace; coal; Industrial Revolution; electrical generation; United States; US; coal power in the United States; environmental health; carbon dioxide; atmosphere of Earth; sulfur dioxide; combustion; acid rain; sulfuric acid; mercury; ton; fossilized; plant; lignin; polymer; cell wall; wood; mechanical properties; Carboniferous; scientist; country; National Science Foundation; Science; wood-decay fungus; white rot fungi; Robert A. Blanchette; Joel A. Jurgens; University of Minnesota; David Hibbett; Professor; Biology; Clark University; fungi; Clark Fungal Database; genomics; Basidiomycetes; Department of Energy; Joint Genome Institute; molecular clock; mutation; organism; energy; biofuel; cellulose; fermentation; sugar; alcohol; yeast; bioremediation; organic molecule; Joseph Spatafora; Oregon State University.