F. Sherwood Rowland
March 15, 2012
I once attended a
conference in
Montreal,
Quebec,
Canada, where I enjoyed a
McDonald's quarter pounder,
avec du fromage. Those were the days! I still attended international conferences, and I could eat
red meat, too!
Montreal is a lovely city. It was the host of the
1976 Summer Olympics, which occurred just a few years before my 1982 visit to attend the
18th IEEE International Magnetics Conference. It was also host to a very important 1987 meeting, the product of which was the
Montreal Protocol. The Montreal Protocol regulated the manufacture of
ozone-depleting substances, specifically certain
chlorofluorocarbons (CFCs).
As a good example of the principle of
unintended consequences, the chemicals responsible for our
Styrofoam coffee cups, and the cool offices in which we drink our hot
coffee, were found to remain in the
atmosphere for many years and react with
ultraviolet radiation from the
sun to produce free
chlorine atoms. The unfortunate consequence of this is that each of these chlorine atoms can catalyze the transformation of many thousands of atmospheric
ozone molecules (O
3) into
oxygen (O
2),
Cl + O3 -> ClO + O3
ClO + O -> Cl + O2
O3 + O -> 2O2
The problem with this
ozone depletion is that our atmospheric ozone protects us from the ultraviolet radiation (UV-B) that causes
skin cancer and
cataracts, and also damages
plants. Eventually, it was shown that
seasonal holes in the ozone layer were found to form, which was alarming enough for the non-scientists to believe in what
scientists were saying, and such chemicals are now regulated.
As could be expected, the producers of CFCs claimed that the science was wrong, there wasn't any problem, and they should continue with business as usual. Parallels to
tobacco regulation and
global warming are not hard to find.
The science, however, was irrefutable, and it led to the award of the
1995 Nobel Prize in Chemistry to
Mario J. Molina and
Frank Sherwood Rowland; and also to
Paul J. Crutzen for his demonstration of the similar damaging effects of
nitric oxide. I'm reminded of
Linus Pauling's campaign against
open-air nuclear testing in the 1950s that won him the
1962 Nobel Peace Prize. Frank Sherwood ("Sherry") Rowland died on March 10, 2012, at age 84.[1-11]

F. Sherwood Rowland
June 28, 1927 - March 10, 2012.
(University of California, Irvine, image by Steve Zylius))
Rowland was born in
Delaware, Ohio, to a father who was a
mathematics professor at
Ohio Wesleyan University, and a mother who taught
Latin. [4,7,9] As is typical among famous scientists, Rowland was a precocious student, graduating
high school at age 15 and entering Ohio Wesleyan at age 16 as a
chemistry student.[9] His education was disrupted when he served in the
Navy as a
radar instructor during
World War II, but he graduated and entered the
University of Chicago as a student of
Willard Libby.[1] At Chicago, he took courses from such luminaries as
Harold Urey and
Enrico Fermi.[4,9]
Chicago awarded Rowland a
Ph.D. for a
thesis in
radiochemistry. After time as a chemistry instructor at
Princeton University, and a professorship at the
University of Kansas, he became founding chairman of the
Department of Chemistry of the
University of California-Irvine when it opened in 1965.[1,7,9]
Rowland's ascent into fame and controversy began with his joint publication of a 1974 paper in
Nature with Molina, who was his
post-doctoral associate at Irvine.[10] This paper identified the CFC-ozone problem, and it caused much consternation in the CFC industry. CFCs were very useful, since they were
nontoxic,
nonflammable, and extremely
stable. They were used as the
refrigerant in air conditioners and refrigerators, and as propellants in
spray cans.[4]
Rowland and Molina realized that their findings were not just of
academic interest. Rowland was so concerned that he told his wife that "It looks like the end of the world."[4,6,7] His wife threw out every spray can,[1] which wouldn't have helped, but I'm sure she refrained from purchasing any more.
Rowland went public, and
lobbied to get CFCs banned. After a 1976 report by the
National Academy of Sciences that verified the problem, the US government banned CFCs in aerosol cans.[7] It wasn't until the discovery of a gaping
ozone hole over the
Antarctic, and a smaller depletion over the
Arctic, that other countries followed suit, leading to the Montreal Protocol.[7,8] Today, CFC production has been reduced by more than 95%.[1]
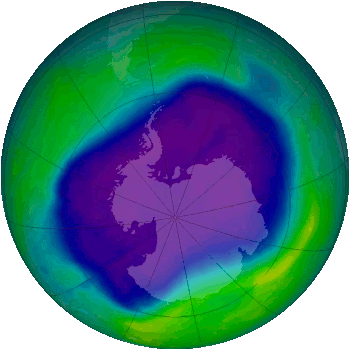
The Antarctic ozone hole, as determined by NASA satellite observations, September 24, 2006.
blue and purple colors are where there is the least ozone.
(Via Wikimedia Commons))
It was tough-going for Rowland and Molina, since they were fighting an industry with $28 billion in annual revenue. They were branded as crazy, or as
communist agents intent on destroying US industry.[3,7] Rowland wasn't invited to speak at any chemistry department from about 1975 to 1985,[3] but he persevered. At a 1997
White House conference on
climate change, he said,
"Is it enough for a scientist simply to publish a paper? Isn't it a responsibility of scientists, if you believe that you have found something that can affect the environment, isn't it your responsibility to actually do something about it, enough so that action actually takes place? If not us, who? If not now, when?"[1,5]
Rowland taught
freshman chemistry at Irvine until he was 80, maintaining an office in a building that was named in his honor.[1,6] He had 425 career publications,[1] was elected to membership in the National Academy of Sciences in 1978, and he served as its foreign secretary from 1994-2002.[11] Rowland was a part of the study of the aftermath of the
Deepwater Horizon oil spill.[6]
Rowland was a modest man.
Ralph J. Cicerone, President of the National Academy of Sciences, recalled that when Rowland was asked about his feelings at winning the Nobel Prize, Rowland said that when you make a big discovery, it means that either the other guy was wrong, or he missed something important. How do you think that makes them feel? [9] Rowland's wife, Joan, has asked that donations be made to the F. Sherwood Rowland Chair and Graduate Fellowship Fund of the UC Irvine Department of Chemistry.[1]
Said
Kenneth Janda, Dean of Physical Sciences at Irvine,[1]
"He saved the world from a major catastrophe: never wavering in his commitment to science, truth and humanity, and did so with integrity and grace."
References:
- Janet Wilson, "UCI Nobel Laureate F. Sherwood Rowland has died at 84," University of California at Irvine Press Release, March 11, 2012.
- Daniel Cressey, "Sherwood Rowland, colossus of atmospheric chemistry, dies," Nature News Blog, March 12, 2012.
- Felicity Barringer, "F. Sherwood Rowland, Cited Aerosols' Danger, Is Dead at 84," The New York Times, March 12, 2012.
- T. Rees Shapiro, "F. Sherwood Rowland, Nobel Prize winner, dies at 84," Washington Post, March 12, 2012.
- Eyder Peralta, "F. Sherwood Rowland, Who Warned Of Thinning Ozone, Has Died," NPR, March 12, 2012.
- Stephen Miller, "Sherwood Rowland 1927-2012 - Chemist Discovered Threat to Ozone Layer," Wall Street Journal Online, March 12, 2012.
- Sherwood Rowland, Telegraph (UK), March 12, 2012
- Ozone pioneer Rowland dies at 84, BBC News, March 12, 2012.
- Shari Roan (Los Angeles Times), "Obituary: F. Sherwood Rowland / Nobel winner who warned of CFCs' danger to ozone," Pittsburgh Post Gazette, March 13, 2012.
- Mario J. Molina and F. S. Rowland, "Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone," Nature, vol. 249, no. 5460 (June 28, 1974), pp. 810-812.
- Nobel Laureate and NAS/IOM Member "Sherry" Rowland Has Died at Age 84, National Academy of Sciences Press Release, March 12, 2012.
Permanent Link to this article
Linked Keywords: Scientific conference; Montreal; Quebec; Canada; McDonald's; quarter pounder; red meat; 1976 Summer Olympics; 18th IEEE International Magnetics Conference; Montreal Protocol; ozone-depleting substances; chlorofluorocarbons; unintended consequences; Styrofoam; coffee cups; coffee; atmosphere; ultraviolet radiation; sun; chlorine; atom; ozone molecule; oxygen; ozone depletion; skin cancer; cataract; plant; season; holes in the ozone layer; scientist; tobacco regulation; global warming; 1995 Nobel Prize in Chemistry; Mario J. Molina; Frank Sherwood Rowland; Paul J. Crutzen; nitric oxide; Linus Pauling; campaign; Partial Nuclear Test Ban Treaty; nuclear testing; 1962 Nobel Peace Prize; Steve Zylius; Delaware, Ohio; mathematics; professor; Ohio Wesleyan University; Latin; high school; chemistry; Navy; radar; World War II; University of Chicago; Willard Libby; Harold Urey; Enrico Fermi; Doctor of Philosophy; Ph.D.; thesis; radiochemistry; Princeton University; University of Kansas; Department of Chemistry; University of California-Irvine; Nature; post-doctoral associate; nontoxic; nonflammable; stable; refrigerant; spray can; academic; lobbying; National Academy of Sciences; ozone hole; Antarctic; Arctic; NASA; Wikimedia Commons; communist; White House; climate change; freshman; Deepwater Horizon oil spill; Ralph J. Cicerone; Kenneth Janda.