Carbon Nano-Aerogel
March 14, 2011
A research article on
carbon nanotube aerogels was published with little fanfare in
ACS Nano in December, 2010.[1] This article has recently gotten some attention in Internet postings, since it's been linked to possible medical and energy applications.[2-4]
Love may make the world go 'round,
science may explain why the world goes around, but applications of science are nice to have around. There are certainly more
cellphones and
iPods than
periodic tables in students' book bags.
The article, by an
interdisciplinary team of
chemists,
materials scientists and
physicists at the
University of Central Florida (
Orlando, Florida), describes a method for making aerogels with multiwalled carbon nanotubes (MWCNTs). I briefly described aerogels in a
previous article (Gel Filtration, August 02, 2007). A
regular gel, as in last night's
gelatin dessert, is a
colloid of interconnected particles "floating" in a liquid medium. In the case of the gelatin dessert, these are
protein molecules in water. Gels are interesting, since they have a density close to that of their liquid, but
mechanical properties like solids.
Aerogels are gels in which the liquid has been replaced by a gas, making them extremely lightweight. The most common use for such materials is as
thermal insulators, and their lightness makes them suitable for many
aerospace applications. They helped to insulate the
Mars Rovers. Aerogels were used as a capture medium for
interplanetary dust in the
NASA Stardust spacecraft. The aerogel detector, a portion of which is shown in the photograph, was made from ninety blocks of
silica aerogel with 99.8% empty space and 0.01% the density of
silica glass.
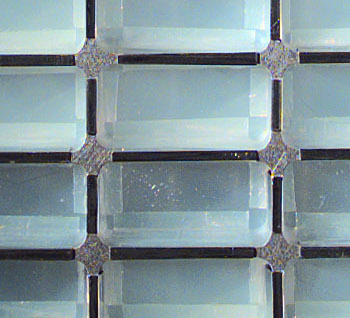
(NASA Image)
As can be imagined, there are quite a few applications for a functional material that's mostly empty space. An aerogel is used as a
Cherenkov radiation detector in the
Belle Experiment. One side effect of the empty space of aerogels is the high surface area of the solid phase. Carbon aerogels are excellent
electrodes for
electric double-layer capacitors (supercapacitors).
The intent of the University of Central Florida research team was to make a MWCNT aerogel with a large
electrical conductivity. Theory suggested that an enhanced
interaction potential between the nanotubes would lower the concentration needed for
percolation; that is, the point at which
electrons will jump between individual tubes. They found that poly(3-(trimethoxysilyl)
propyl methacrylate) allows formation of bonds between the nanotubes. Subsequent removal of the liquid phase created a free-standing aerogel with a density of just 4 mg/cm
3.
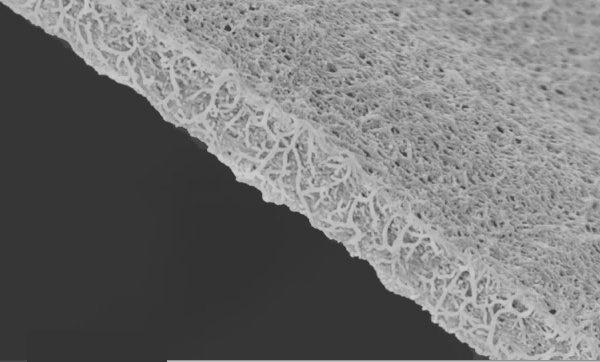
Photomicrograph of a multiwalled carbon nanotube aerogel. The scale of the image is a few micrometers across (UCF photograph).
As shown in the above photomicrograph, the nanotubes with 100 nm walls were separated by voids that were about 50-150 μm in size. The surface area was measured as 580 m
2/g. The MWCNT aerogels were able to withstand many
mechanical compression cycles, and they had an electrical conductivity of 3.2 x 10
-2 S·cm
-1. It was found that an
electrical current pulse would increase the conductivity to 0.67 S·cm
-1.
The material could be reversibly compressed to just 5% of its volume, for at least a thousand cycles.[2] Most significantly, the conductivity was highly sensitive to
pressure,[2] much like the
carbon button microphones I
wrote about earlier (Piezomolecular Effect, February 22, 2011). The large surface area, combined with its intrinsic conductivity, indicates a possible use as a
chemical sensor.[3] Since this material is essentially rarefied
carbon, it's been suggested that the name, "frozen smoke," may be most appropriate.[4]
References:
- Jianhua Zou†, Jianhua Liu, Ajay Singh Karakoti, Amit Kumar, Daeha Joung, Qiang Li, Saiful I. Khondaker, Sudipta Seal and Lei Zhai, "Ultralight Multiwalled Carbon Nanotube Aerogel," ACS Nano, vol. 4, no. 12 (December 28, 2010), pp. 7293-7302.
- Stewart Bland, "Solid smoke," Materials Today, March 1, 2011
- Zenaida Gonzalez, "New 'Frozen Smoke' May Improve Robotic Surgery," University of Central Florida Press Release, March 1, 2011
- Timon Singh, "Super Batteries Made From ‘Frozen Smoke’ May be Here Soon," Inhabitat.com, March 2, 2011
Permanent Link to this article
Linked Keywords: Carbon nanotube; aerogel; ACS Nano; Love sculpture; Robert Indiana; Love; science; cellphone; iPod; periodic table; interdisciplinarity; interdisciplinary; chemist; materials scientist; physicist; University of Central Florida; Orlando, Florida; gel; gelatin dessert; colloid; protein molecule; mechanical properties; thermal insulator; aerospace; Mars Rovers; cosmic dust; interplanetary dust; NASA; Stardust spacecraft; silica; silica glass; Cherenkov radiation; Belle Experiment; electrode; electric double-layer capacitor; electrical conductivity; interaction potential; percolation; electron; propyl; methacrylate; poly(3-(trimethoxysilyl) propyl methacrylate); mechanical compression; Siemens; electrical current; pressure; carbon button microphone; chemical sensor; carbon.